Abstract
Purpose
To evaluate whether anti-CTLA-4 therapy could suppress residual tumor progression and improve survival after insufficient radiofrequency ablation (RFA) in a subcutaneous murine hepatocellular carcinoma (HCC) model.
Materials and Methods
Forty mice with tumors established on their right flanks were randomly divided into four groups: control group (no treatment), RFA group (insufficient RFA alone), anti-CTLA-4 group (anti-CTLA-4 monotherapy), and RFA + anti-CTLA-4 group (insufficient RFA + anti-CTLA-4). In each group, eight mice were assessed for residual tumors and survival; another two mice were killed on day 14 for histopathologic studies. On day 42, a re-challenge test was performed in the survived mice of RFA + anti-CTLA-4 group to determine whether systemic anti-tumor immunity was established.
Results
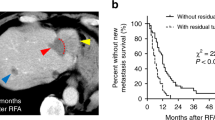
The specific growth rate of residual tumors was significantly less in RFA + anti-CTLA-4 group than that of the other three groups (all p < 0.05). The disease control rate was 50% in RFA + anti-CTLA-4 group, while no animals in the other three groups showed disease control. Animals in RFA + anti-CTLA-4 group had longer survival times than those in the other three groups (all p < 0.05). Expression of CD4+ lymphocytes in residual tumors and IFN-γ production in response to H22 tumor cells were significantly higher in RFA + anti-CTLA-4 group than those in the other three groups (all p < 0.05). Three of the five survived mice in RFA + anti-CTLA-4 group underwent tumor re-challenge exhibited tumor rejection.
Conclusions
The present study demonstrated that CTLA-4 blockade injection could suppress the growth of residual tumors and improve survival after insufficient RFA in a subcutaneous murine HCC model.






Similar content being viewed by others
References
Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569–77.
Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–7.
Ahmed M, Brace CL, Lee FT, et al. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–69.
Yan K, Chen MH, Yang W, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol. 2008;67:336–47.
McWilliams JP, Yamamoto S, Raman SS, et al. Percutaneous ablation of hepatocellular carcinoma: current status. J Vasc Interv Radiol. 2010;21:S204–S213213.
Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684–92.
Waki K, Aikata H, Katamura Y, et al. Percutaneous radiofrequency ablation as first-line treatment for small hepatocellular carcinoma: results and prognostic factors on long-term follow up. J Gastroenterol Hepatol. 2010;25:597–604.
Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900–9.
Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Interv Radiol. 2013;36:166–75.
Okusaka T, Okada S, Ueno H, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95:1931–7.
Kim YS, Lim HK, Rhim H, et al. Ablation of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2014;28:897–908.
Kong J, Kong J, Pan B, et al. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1alpha/VEGFA. PLoS ONE. 2012;7:e37266.
Ahmed M, Kumar G, Moussa M, et al. Oncogenesis: an “off-target” effect of radiofrequency ablation. Radiology. 2015;276:426–32.
Shi L, Wang J, Ding N, et al. Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat Commun. 2019;10:5421.
Den Brok MHMGM, Sutmuller RPM, Van Der Voort R, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–9.
Lemdani K, Mignet N, Boudy V, et al. Local immunomodulation combined to radiofrequency ablation results in a complete cure of local and distant colorectal carcinoma. Oncoimmunology. 2019;8:1550342.
Mehta A, Oklu R, Sheth RA. Thermal ablative therapies and immune checkpoint modulation: can locoregional approaches effect a systemic response? Gastroenterol Res Pract. 2016;2016:9251375.
Slovak R, Ludwig JM, Gettinger SN, et al. Immuno-thermal ablations—boosting the anticancer immune response. J Immunother Cancer. 2017;5:78.
Minami Y, Nishida N, Kudo M. Radiofrequency ablation of liver metastasis: potential impact on immune checkpoint inhibitor therapy. Eur Radiol. 2019;29:5045–51.
Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–51.
Xie C, Duffy AG, Mabry-Hrones D, et al. Tremelimumab in combination with microwave ablation in patients with refractory biliary tract cancer. Hepatology. 2019;69:2048–60.
Watanabe H, Okada M, Kaji Y, et al. New response evaluation criteria in solid tumours—revised RECIST guideline (version 1.1). Jpn J Cancer Chemother. 2009;36:2495–501.
Hamamoto S, Okuma T, Yamamoto A, et al. Radiofrequency ablation and immunostimulant OK-432: combination therapy enhances systemic antitumor immunity for treatment of VX2 lung tumors in rabbits. Radiology. 2013;267:405–13.
Kageyama K, Yamamoto A, Okuma T, et al. Radiofrequency ablation of liver tumors in combination with local OK-432 injection prolongs survival and suppresses distant tumor growth in the rabbit model with intra- and extrahepatic VX2 tumors. Cardiovasc Interv Radiol. 2013;36:1383–92.
Waitz R, Solomon SB, Petre EN, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012;72:430–9.
Showalter A, Limaye A, Oyer JL, et al. Cytokines in immunogenic cell death: applications for cancer immunotherapy. Cytokine. 2017;97:123–32.
Haen SP, Pereira PL, Salih HR, et al. More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol. 2011;2011:160250.
Chen L, Sun J, Yang X. Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: current status. Cancer Lett. 2016;370:78–84.
Yu Z, Geng J, Zhang M, et al. Treatment of osteosarcoma with microwave thermal ablation to induce immunogenic cell death. Oncotarget. 2014;5:6526–39.
Adkins I, Sadilkova L, Hradilova N, et al. Severe, but not mild heat-shock treatment induces immunogenic cell death in cancer cells. Oncoimmunology. 2017;6:e1311433.
Sharma P, Allison JP. The future of immune checkpoint therapy. Science (80-). 2015;348:56–61.
Greten TF, Mauda-Havakuk M, Heinrich B, et al. Combined locoregional-immunotherapy for liver cancer. J Hepatol. 2019;70:999–1007.
Kim YS, Lim HK, Rhim H, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89–97.
Funding
This study was funded by Shanghai Anticancer Association EYAS PROJECT (Grant Number SACA-CY1C17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed Consent
For this type of study, informed consent is not required.
Consent for Publication
For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, L., Wang, J., Jiang, J. et al. CTLA-4 Blockade Suppresses Progression of Residual Tumors and Improves Survival After Insufficient Radiofrequency Ablation in a Subcutaneous Murine Hepatoma Model. Cardiovasc Intervent Radiol 43, 1353–1361 (2020). https://doi.org/10.1007/s00270-020-02505-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-020-02505-6