Abstract
Purpose
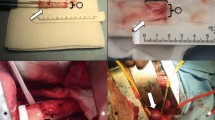
To investigate the technical feasibility of a novel exoskeleton Seal® stent-graft and analyze early histologic changes in the porcine abdominal aorta.
Materials and Methods
Six pigs received an abdominal stent-graft (Group I), and six received an iliac branch stent-graft (Group II). Groups were subdivided as follows: Group Ia, which received three bifurcated main-body stent-grafts; Group Ib, which received three bifurcated main-body stent-grafts with both iliac graft-stents; Group IIa, which received three simple uni-iliac tapered stent-grafts; and Group IIb, which received three uni-iliac tapered tapered stent-grafts with right straight limb and left branched limb. Statistical analyses were performed with the Wilcoxon signed-rank test and mixed-model regression analysis.
Results
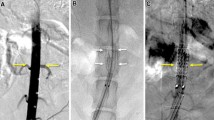
The primary technical success rate (< 24 h) was 83% because of two acute thromboses in the lumen of the stented abdominal aorta immediately after stent-graft placement. At 4 weeks, late thrombosis occurred in two pigs. Higher mean neointimal hyperplasia areas (23.5% vs. 16.2%; P = .047), neointimal hyperplasia thicknesses (545.5 μm vs. 422.2 μm; P = .001), and degrees of collagen deposition (2.71 vs. 2.33; P = .002) were observed at the bare-metal stent-graft compared with the proximal exoskeleton portion of the stent-graft, with no significant differences between the patent and occluded groups or among the four types of stent-grafts.
Conclusions
The exoskeleton stent-graft demonstrates 66% of patency rate during 1-month follow-up due to four cases of thromboses; however, the endothelialization on the junction of proximal graft showed no significant differences between the patent and occluded groups. Further studies should investigate long-term outcomes with prolonged neointimal hyperplasia.







Similar content being viewed by others
References
Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–9.
Bashar AH, Kazui T, Terada H, Suzuki K, Washiyama N, Yamashita K, et al. Histological changes in canine aorta 1 year after stent-graft implantation: implications for the long-term stability of device anchoring zones. J Endovasc Ther. 2002;9:320–32.
Wu MH, Shi Q, Onuki Y, Kouchi Y, Sauvage LR. Histologic observation of continuity of transmural microvessels between the perigraft vessels and flow surface microostia in a porous vascular prosthesis. Ann Vasc Surg. 1996;10:11–5.
Spanos K, Karathanos C, Saleptsis V, Giannoukas AD. Systematic review and meta-analysis of migration after endovascular abdominal aortic aneurysm repair. Vascular. 2016;24:323–36.
Kent F, Ambler GK, Bosanquet DC, Twine CP, BSET (British Society for Endovascular Therapy). The safety of device registries for endovascular abdominal aortic aneurysm repair: systematic review and meta-regression. Eur J Vasc Endovasc Surg. 2018;55(2):177–83.
Steuer J, Lachat M, Veith FJ, Wanhainen A. Endovascular grafts for abdominal aortic aneurysm. Eur Heart J. 2016;37(2):145–51.
Maldonado TS, Mosquera NJ, Lin P, Bellosta R, Barfield M, Moussa A, et al. Gore Iliac Branch Endoprosthesis for treatment of bilateral common iliac artery aneurysms. J Vasc Surg. 2018;68:100–8.
Schneider DB, Matsumura JS, Lee JT, Peterson BG, Chaer RA, Oderich GS. Prospective, multicenter study of endovascular repair of aortoiliac and iliac aneurysms using the Gore Iliac Branch Endoprosthesis. J Vasc Surg. 2017;66:775–85.
Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–60.
Li YD, Song HY, Kim JH, Woo CW, Park JH, Kim TH, et al. Evaluation of formation of granulation tissue caused by metallic stent placement in a rat urethral model. J Vasc Interv Radiol. 2010;21(12):1884–90.
Gorin DR, Arbid EJ, D’Agostino R, Yucel EK, Solovay KS, La Morte WW, et al. A new generation endovascular graft for repair of abdominal aortic aneurysms. Am J Surg. 1997;173:159–64.
Deery SE, Shean KE, Pothof AB, O’Donnell TFX, Dalebout BA, Darling JD, et al. Three-year results of the Endurant Stent Graft System Post Approval Study. Ann Vasc Surg. 2018;50:202–8.
Lambert AW, Budd JS, Fox AD, Potter U, Rooney N, Horrocks M. The incorporation of a stent-graft into the porcine aorta and the inflammatory response to the endoprosthesis. Cardiovasc Surg. 1999;7:710–4.
Kim YI, Choi YH, Chung JW, Kim HC, So YH, Kim HB, et al. Tissue responses to stent grafts with endo-exo-skeleton for saccular abdominal aortic aneurysms in a canine model. Korean J Radiol. 2014;15:622–9.
Kim HB, Choi YH, So YH, Min SK, Kim HC, Kim YI, et al. Tissue responses to endovascular stent grafts for saccular abdominal aortic aneurysms in a canine model. J Korean Med Sci. 2012;27:1170–6.
Kim JH, Cho YK, Seo TS, Song MG, Jeon YS, Han YM, et al. Clinical outcomes for endovascular repair of abdominal aortic aneurysm with the Seal stent graft. J Vasc Surg. 2016;64:1270–7.
Calero A, Illig KA. Overview of aortic aneurysm management in the endovascular era. Semin Vasc Surg. 2016;29:3–17.
Yavuz K, Geyik S, Pavcnik D, Uchida BT, Corless CL, Hartley DE, et al. Comparison of the endothelialization of small intestinal submucosa, dacron, and expanded polytetrafluoroethylene suspended in the thoracoabdominal aorta in sheep. J Vasc Interv Radiol. 2006;17:873–82.
Guidoin R, Chakfé N, Maurel S, How T, Batt M, Marios M, et al. Expanded polytetrafluoroethylene arterial prostheses in humans: histopathological study of 298 surgically excised grafts. Biomaterials. 1993;14:678–93.
Sincos IR, da Silva ES, Belczak SQ, Baptista Sincos AP, de Lourdes Higuchi M, Gornati V, et al. Histologic analysis of stent graft oversizing in the thoracic aorta. J Vasc Surg. 2013;58:1644–51.
van der Bas JM, Quax PH, van den Berg AC, van Hinsbergh VW, van Bockel JH. Ingrowth of aorta vascular cells into basic fibroblast growth factor-impregnated vascular prosthesis material: a porcine and human in vitro study on blood vessel prosthesis healing. J Vasc Surg. 2002;36:1237–47.
Funding
The study was supported by a Grant No. 2018-10 from the Kangdong Sacred Heart Hospital Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed Consent
For this type of study informed consent is not required.
Consent for Publication
For this type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, JH., Cho, Y.K., Her, K. et al. Histologic Analysis with the Newly Designed Exoskeleton Seal® Stent-Graft in the Porcine Abdominal Aorta. Cardiovasc Intervent Radiol 42, 1331–1342 (2019). https://doi.org/10.1007/s00270-019-02261-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02261-2