Abstract
Polyandry in social Hymenoptera is associated with reduced within-colony relatedness and increased colony-level ecologic fitness. One explanation for this sees increasing within-nest genetic diversity as a mechanism for improving group task efficiency and colony competitiveness. A queen on her mating flight captures nearly 90% of her breeding population’s allele potential by her tenth effective mating (me ~ 10 males). Under this population allele capture (PAC) model, colony fitness gains track mating number in an asymptotic manner, leveling out after about the tenth mating. A supporting theory we call the genotype composition (GC) model sees genetic novelty at mating levels higher than the me ~ 10 asymptote, the hyperpolyandry zone, resulting from unique genotype compositions whose number are potentially infinite. Colony fitness gains under the GC model will track mating number in a linear manner. We set up field colonies with Apis mellifera queens each instrumentally mated with 1, 2, 4, 8, 16, or 32 males, creating a polyandry gradient bracketing the qualitative divide of me ~ 10, measured tokens of colony level fitness, and collected observation hive data. Our results lead us to conclude that (1) ancestral colony traits fundamental to eusociality (cooperative brood care) respond to mating level changes at or below me ~ 10 in a manner consistent with the PAC model, whereas (2) more derived specialized colony phenotypes (resistance to the non-native parasite Varroa destructor) continue improving with increasing me in a manner consistent with the GC model. By either model, (3) the mechanism for increasing colony fitness is an increase in worker task specialisms and task efficiency.
Significance statement
Polyandry is a female’s practice of mating with many males, storing their sperm, and using it to produce genetically diverse offspring. In complex social bees, a queen captures nearly 90% of her breeding population’s diversity potential by her tenth mating; however, queens in nature routinely mate with many more than ten males. We tested two models that, together, explain how social bee colonies ecologically benefit from queen mating numbers ranging from 2 to potential infinity. A population allele capture (PAC) model focuses on colony fitness gains at mating numbers at or below 10, and we provide evidence that it was at these polyandry levels that significant gains were made in an ancestral eusocial trait, cooperative brood care. A genotype composition (GC) model focuses on colony fitness gains at higher mating numbers, and we believe these gains are centered around more recently evolved ecologic specialisms such as parasite resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyandry, a female’s practice of mating with multiple males, has played an important role in the evolution of complex eusociality in the Hymenoptera. Equipped with a spermatheca that allows her to store sperm from multiple mates, nourish it and use it across her lifetime, a polyandrous queen reduces average relatedness across her offspring, appearing to undermine the foundations of kin-based group formation (Ratnieks and Helanterä 2009). However, at the same time the increase in within-nest genetic diversity increases fitness of the group. One set of hypotheses for explaining these dynamics sees increasing within-nest genetic diversity as a mechanism for improving group task efficiency and colony competitiveness (Crozier and Fjerdingstad 2001; Gove et al. 2009). Other viable hypotheses see polyandry as an adaptation against sperm limitation (Kraus et al. 2004) or inbreeding at sex or other loci (Page 1980), or as a bet-hedging strategy for avoiding phenotypic extremes (Yasui 2001).
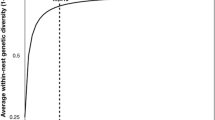
Any hypotheses based on genetic diversity are constrained by the rules of haplo-diploid hymenopteran inheritance. The fraction of alleles shared in common by a mother’s daughters is capped at 75%, and roughly 90% of this potential is achieved by the queen’s tenth effective mating (= me, the queen’s mating number adjusted for relative contribution of each of her mates to actual progeny (Starr 1985)) with only marginal allelic gains thereafter (Palmer and Oldroyd 2000). Under this population allele capture (PAC) model, colony fitness gains track mating number in an asymptotic manner, leveling out after roughly the tenth mating (Fig. 1).
In a colony of social Hymenoptera, average worker relatedness decreases as a function of the queen’s mating number according to the formula r = 0.25 + (0.5/me) where r = relatedness, the probability that any two individuals share the same alleles at any given loci and me = effective mating number, the queen’s mating number adjusted for relative contribution of each of her mates to actual progeny (Starr 1985); by extension, average genetic diversity = 1-r. The most rapid decreases in average worker relatedness happen when the mother increases her mating number from 1 (r = 0.75) to 2 (r = 0.5) to 3 (r = 0.42), and so forth in a geometric fashion until me∼10 with only marginal decreases thereafter (Palmer and Oldroyd 2000)
A supporting theory we call the genotype composition (GC) model sees genetic novelty at mating levels in the hyperpolyandry zone, those > me ~ 10, arising from unique genotype compositions, expressed as optimum ratios of specialists and non-specialists. The number of these compositions is potentially infinite because many compositions are possible at the same value of average relatedness (Fuchs and Moritz 1999). Colony fitness gains under the GC model, therefore, will track mating number in a linear manner. Polyandry in social Hymenoptera must therefore be understood as a continuum along which different explanations are required depending on whether the species’s mating biology falls below or above the me∼10 asymptote. The Apini and its sole genus Apis brackets this divide, with average me ranging from 5.6 in A. florea (Oldroyd et al. 1995) to over 44 in A. dorsata (Tarpy et al. 2004) with A. mellifera, our model species, ranging from 11 (Simone-Finstrom and Tarpy 2018) to over 20 (Kraus et al. 2005) with records of colony subfamily representation as high as 77 (Withrow and Tarpy 2018). It should be noted that Apis expresses some of the highest hyperpolyandry levels known, matched only by African army ants Dorylus (Anomma) molestus me = 17.5 (Kronauer et al. 2004) and neotropical army ants me < 25 (Barth et al. 2014).
For most of the history of evolutionary biology, the high ground for explaining character state change has been held by gradualism. Polyandry certainly seems compatible with this notion, with first two mates, then three and so forth, with adaptive colony-level changes obtaining along the way. We cannot rule out saltational leaps (Katsnelson et al. 2019; Bakhtin et al. 2021), especially given that hyperpolyandrous mating numbers could be stochastic outcomes of the chaotic and populous drone congregation areas that young queens visit during their aerial mating flights (Tarpy and Page 2000). But theory (Fuchs and Moritz 1999) and experimental evidence (Delaplane et al. 2015, 2021) show that hyperpolyandry confers direct colony-level adaptive benefits especially for more derived tasks such as parasite resistance.
In most honey bee experiments, investigators have compared colony effects among queen bees instrumentally inseminated with 1 or more drones (= mo, observed mating number). An overwhelming majority of these studies (Tarpy 2003; Tarpy and Seeley 2006; Mattila and Seeley 2007, 2010, 2011, 2014; Seeley and Tarpy 2007; Mattila et al. 2008; Eckholm et al. 2011, 2015; Girard et al. 2011; Desai and Currie 2015) has compared colony effects of some observed mating number (mo > 1) against only one other class – a single-drone insemination (mo = 1). This approach is useful for demonstrating qualitative differences between polyandry and monandry but offers little understanding of the variation that occurs along a continuum of mating levels that could drive a gradualistic process toward the mating numbers we see today.
In this paper, we are interested in describing colony-level changes that occur along a range of queen mating levels below and above the me∼10 asymptote, including low mating numbers of the kind we expect at incipient stages of polyandry in the corbiculate ancestors of Apini and hyperpolyandrous levels we assume are derived. We hypothesize that (1) colony-level gains in an ancestral task foundational to eusociality (cooperative brood care) will track the geometric increase and plateau of within-colony genetic diversity illustrated in Fig. 1 consistent with the PAC model, whereas (2) colony fitness gains in a more derived character (resistance to a non-natural parasite) will not be constrained by the rules of hymenopteran inheritance and are instead free to increase linearly in compliance with the GC model. As either scenario collapses to gene-based behavioral or physiological effects in workers, we predict that (3) changes in polyandry state will be reflected in detectable changes in worker task distributions. Although fitness in the honey bee is formally the number of swarms produced and the number of drones that successfully mate, we use two tokens for colony fitness, brood production and colony Varroa mite levels, that are reliable predictors of colony winter survival and reproduction (Seeley and Visscher 1985; Van Dooremalen et al. 2012). Moreover, the human-facilitated spread of the parasitic mite Varroa destructor on its new host Apis mellifera makes a good model system for studying how A. mellifera responds to an idiosyncratic stressor with which it has had an extremely brief evolutionary history (Rosenkranz et al. 2010).
Methods
Study area and general design
The experiment involved instrumentally inseminating virgin queens at different mating levels, housing each in a field colony, and subsequently tracking colonies for brood production and parasitic mite levels. The study was conducted near Athens, Georgia, USA during spring and summer 2019 into spring of 2020. Experimental field colonies were started between 20–21 Mar 2019, each with a standard package of 3 lb (1.4 kg) worker bees housed in a single deep Langstroth super with a surrogate open-mated queen who was removed and replaced after experimental queens became available. Forty virgin supersister queens, all sisters of one queen inseminated with one male such that all shared the same mother and father, were reared, caged, and individually housed in one of the field colonies after its surrogate queen was removed. Inseminations began 15 Apr 2019, and each inseminated queen was returned to her respective colony so we could subsequently track her colony’s brood measures. All colonies were kept in one apiary.
With an ancillary observation hive study, we tracked same-aged worker cohorts through their task careers with the aim of identifying differences in task distribution based on polyandry.
Instrumental inseminations
Experimental queens were each instrumentally inseminated (Cobey et al. 2013) with a mixture of physiological saline (15% of volume) and the semen of 1, 2, 4, 8, 16, or 32 drones. Semen batches collected from multiple drones were each discharged into an Eppendorf tube, manually mixed with saline to encourage equal sperm representation per drone, and redrawn into the same syringe subsequently used to inseminate queens. As our independent variable was polyandry sensu stricto and not drone source genetics, we randomly selected 10 non-experimental colonies to serve as drone donors. These drone-source colonies were from common commercial beekeeping stocks but otherwise genetically uncharacterized. Our different treatments were each a mixture of semen from a random sampling of drones from the 10 colonies (or from one colony in the case of single-inseminated queens). Each queen received a mixed semen volume of ~ 4 µL, controlling for effects of semen volume (Payne and Rangel 2018). Following insemination, each queen was marked with a unique number and returned to her host colony. Over the next two days, each queen was treated with CO2 for 10 min to stimulate egg laying (Mackensen 1947).
Colony management
Queens were released after 1–3 days and colonies managed according to standard practices. Colonies were fed supplemental sugar solution and given honey supers as needed to accommodate colony growth. After all queens were released and before we collected colony level data, colonies were maintained for another 5 weeks to monitor for normal queen egg laying performance and to allow for population turnover to progeny of test queens. Following this period of attrition, the beginning number of queens/colonies for each insemination group was mo = 1 (n = 6 colonies), mo = 2 (7 colonies), mo = 4 (6 colonies), mo = 8 (7 colonies), mo = 16 (8 colonies), and mo = 32 (6 colonies). This ca. 35-day delay means that an adult bee reared from an egg laid by its mother on her day of release will be entering into her peak nursing period in the honey bee’s schedule of age-based tasks, according to a colony growth model developed for our region (Bartlett et al. 2022). This also means that experimental polyandry effects were stronger later in our sampling regimen than earlier.
Dependent variables
Brood production and parasite numbers
Holistic colony strength measures, considered proxies for fitness, were taken at roughly monthly intervals in Jun, Jul, Aug, Sep, and Oct 2019.
Brood area was derived by visually summing proportions of whole deep frames covered by brood (Delaplane et al. 2013) and converting frames of brood to cm2 by the observation that one deep Langstroth comb (both sides) = 1760 cm2.
Relative numbers of parasitic Varroa destructor mites were found by inserting sticky sampling sheets into bottom board hive inserts and recording the number of Varroa mites trapped after 24 h (Dietemann et al. 2013).
Worker task distributions
In Mar 2020, we removed combs of emerging brood from one overwintered experimental field colony whose queen was mated at the mo = 16 level and from another colony whose queen was mated at the mo = 32 level. These were the only two surviving colonies with enough brood to perform the experiment. These combs were labeled by colony of origin, bagged individually, and kept overnight in an incubator at 35 °C. The next morning, 100 newly emerged worker bees from each colony were individually numbered with thoracic tags (Mann Lake) color-coded by colony of origin, and all 200 workers introduced to one observation bee hive (day = 0) with a non-experimental open-mated queen and 311 of her daughter workers whose number was estimated from a photograph. The resident workers were therefore surrogate hosts to two embedded cohorts for whom we have knowledge of mo. The disadvantage of this arrangement is that it is devoid of the complexity of thigmotaxic and social cues that govern polyandry effects in a normal whole colony. The advantage is that it allows us to compare how different polyandry levels can capture and express specialisms in a common experimental environment. The comb in the observation hive was 1-sided to restrict bees to one surface and ease observations. Beginning on day = 1 (27 Mar 2020) and for 18 more days until 14 Apr 2020 one investigator, naïve to the bee labeling designations, observed the marked cohort through the glass for 15 min and recorded each incidence when a focal bee performed one of 18 unambiguously identifiable behaviors: tending queen, watching recruitment dance, dancing, administering vibration dance, receiving vibration dance, shaping cappings, shaping cell edges, receiving grooming, autogrooming, allogrooming, pollen foraging (possessing pollen on its corbiculum), building comb, fanning entrance, engaging in trophallaxis, capping cells, cleaning emerged brood cells, trimming cappings, and antennating another bee.
Statistical analyses
Strength measures
Analyses were performed using SAS v. 9.04 and JMP Pro v. 11.0.0. Observations for colony strength were taken from each colony at 5 different timepoints (Jun, Jul, Aug, Sep, Oct). We were interested in determining the effect of polyandry over time on two tokens of colony fitness – brood area and mite count. Our assumption was that increasing mating numbers would be associated with improving colony level fitness.
When residuals were found to deviate from normal distribution, the dependent data were log(ln) transformed to restore normality and confirmed as such with Shapiro–Wilk or Kolmogorov–Smirnov tests. We used repeated measures models where each colony is measured at every timepoint and colony is considered a random effect. Random intercepts were included to allow for colonies with the same mating level to be different from each other. All models include polyandry and time; interactions of polyandry and time (which would show whether the effect of polyandry was different at different timepoints) were tested for each dependent variable but found non-significant in every case and dropped. Polyandry was considered numerically (value = mo), categorically, or as ranks such that (mo, rank) were (1, 1), (2, 2), (4,3), (8,4), (16,5), or (32,6), respectively. Linear as well as quadratic (curved) relationships of polyandry to outcomes were considered when polyandry was treated numerically or as ranks. When polyandry was considered categorically, we assumed no functional relationship between outcomes and increasing levels of polyandry; each level was simply allowed to be different from another. Final models were chosen based on their lower AIC and BIC values compared to alternative polyandry treatments.
Worker task distributions
Assuming that increasing polyandry and colony diversity are associated with changes in worker task specialization leading to changes in colony fitness (Nonacs and Kapheim 2007; Gove et al. 2009), we considered two hypotheses: (1) Increasing polyandry (mo = 16 vs. 32) is associated with higher number of specialists identifiable as individuals who persist longer (across days) at certain tasks over others and (2) Increasing polyandry is associated with decreasing numbers of overachievers identified as individuals who perform a higher than average number of tasks (fewer generalists). In the end we did not have enough workers persisting in any task long enough to confidently label as specialists, so we settled on hypothesis #2 which focuses on the number of tasks per bee. We consider recovery of bees good. Of the 100-polyandry mo = 16 cohort, 11 bees were never observed, leaving 89 bees to include in the analysis. Of the 100 mo = 32 cohort, 6 were never observed, leaving 94 bees to consider in the analysis.
We tested for overachieving bees two ways: (1) by calculating average number of tasks performed per individual per day observed and (2) by calculating average number of unique tasks performed per individual per day.
On each day a bee was observed, the number of non-resting tasks it performed was counted. If a bee was seen performing the same task more than once during the same observation period, it was counted as one task. If a bee was not observed during the observation on a particular day, that day was not counted in the analysis for that bee. The parameter was converted to number of tasks per observation day. As an example, if a bee was seen performing two tasks on one day, one task on one other day, and was not observed any other days, the total number of days observed is 2, and the total number of tasks is 3 and tasks per observation day is 3/2 = 1.5.
To compare these averages for the two mating levels, we used a Poisson regression. This is a count model that considers the total number of tasks a bee was seen performing (adding together the count of different tasks on each occasion). Variability across bees was shown to be lower than expected for a Poisson regression. Therefore, the model was adjusted for underdispersion by decreasing standard errors on the basis of a Pearson chi-square measuring the extent of the underdispersion.
The second analysis considers the number of unique tasks performed per bee over the course of the study. For example, if a bee performs two tasks on one day and one of the tasks again on a second day, this counts as just two kinds of tasks performed, and average unique tasks performed per observation day is 2/2 = 1. To compare these averages for the two mating levels, we used a Poisson regression as before, once again adjusting for underdispersion in response to lower variability across bees than expected.
Results
Brood area
After initial modeling, the residuals from the brood area model were shown to be acceptably normally distributed with both Shapiro–Wilk and Kolmogorov–Smirnov tests.
Polyandry was at first treated categorically, and only timepoint was found to be statistically significant. Post-hoc contrasts were run to see if there were polynomial trends across the levels of polyandry. It was found that there was at least a linear trend, so polyandry was next treated as a numeric variable. Both the actual value of polyandry (mo) and the rank value of polyandry were used as numeric independent variables in separate models. Polyandry was found to have significant curvature in its relationship with brood area. The final model, chosen because it had the lower AIC and BIC values of the two, included numeric polyandry (not rank) as a continuous variable and detected a linear effect of polyandry (F1, 32 = 7.47; P = 0.0101), a quadratic effect of polyandry (F1, 31.9 = 5.39; P = 0.0268), and an effect of timepoint (F4, 127 = 61.8; P < 0.0001). From Fig. 2, we see that brood area increases with mating number until it reaches its estimated peak at polyandry level ~ mo = 20 after which it declines. Brood area declined over timepoints, and changes in brood area expressed consistently over time across polyandry levels as the two terms did not interact (Fig. 2).
Changes in brood area (cm2) in honey bee colonies whose queens were inseminated with mo = 1, 2, 4, 8, 16, or 32 males. Data were collected in monthly timepoints from Jun-Oct. Pooling all timepoints, changes in brood area were explained by the quadratic equation y = 1974 + (101.4*mo)-(2.56* mo2). Data are color-coded according to timepoint, and brood area significantly decreased over time except for spikes in June and September. Tukey’s tests of lsmeans showed that all timepoints differed from one another at the 0.05 level except for Jun and Jul
Mite counts
After initial modeling, the residuals from the mite count model were found to be right skewed. A natural log (ln) transformation of mite count alleviated this; residuals tested normally distributed with both Shapiro–Wilk and Kolmogorov–Smirnov tests.
At first, polyandry was treated categorically and only time was shown to be statistically significant in this model. Despite the overall lack of significance, statistical contrasts were run as post-hoc tests to see if there were polynomial trends across levels of polyandry (for example, whether there was a linear or quadratic (curved) relationship of polyandry with ln(mite count)). It was found that there was a linear trend, so polyandry was next treated as a numeric variable. Both the actual value of polyandry and the rank value of polyandry were used as numeric independent variables in separate models. Neither had a statistically significant quadratic (curvature) term in relationship to ln(mite count). The final linear model, chosen because it had the lower AIC and BIC values of the two, used polyandry rank as a continuous variable and found significant effects for both polyandry rank (F1, 38.2 = 5.07; P = 0.0302) and timepoint (F4, 133; = 29.6; P < 0.0010). The linear coefficient for ranked polyandry across all timepoints was significantly negative, -0.156 ± 0.069 (coefficient ± SE; t-2.25 = 38.1; P = 0.0302), indicating that as polyandry increased by one rank, from 1 through 6, the ln(mite count) decreased by an estimated -0.156. The ln(mite count) increased over time but performed consistently without interacting across polyandry levels (Fig. 3).
Changes in natural log-transformed V. destructor mite counts in honey bee colonies whose queens were inseminated with mo = 1, 2, 4, 8, 16, or 32 males. Polyandry was ranked for this analysis such that (mo, rank) was (1, 1), (2, 2), (4,3), (8,4), (16,5), and (32,6), respectively. Data were collected in monthly timepoints from Jun-Oct. Pooling all timepoints, for every increase in rank polyandry from 1 to 6, ln(mite count) decreased according to the linear equation y = 3.6-(0.156*rank mo). Data are color-coded according to timepoint, and mite counts significantly increased over time but never interacted with ranked polyandry. Tukey’s tests of lsmeans showed that timepoints not differing significantly from one another at the 0.05 level were Jun and Jul; Aug and Sep; and Sep and Oct
Worker task distributions
The Poisson regression likelihood ratio tests found significant differences in polyandry treatments for worker tasks performed per day (F1,181 = 5.01, P = 0.0253) which was significantly higher in the mo = 16 group (0.725 ± 0.031 tasks per worker per day; mean ± SEM) than the mo = 32 group (0.632 ± 0.028) (Fig. 4). The same held true when the effect was considered as unique worker tasks performed per day (F1,181 = 5.79, P = 0.0171) which was significantly higher in the mo = 16 group (0.604 ± 0.027 unique tasks per worker per day; mean ± SEM) than the mo = 32 group (0.517 ± 0.025) (Fig. 5).
Discussion
Our results show that by increasing her mating number mo (a proxy for me), a honey bee queen confers on her colony improved brood production and slower population growth of a dangerous non-natural parasite, the mite Varroa destructor. These results were accompanied by a demonstration that increasing queen mating number from mo = 16 to = 32 is associated with daughter cohorts who perform comparatively fewer tasks and fewer kinds of tasks per observation interval. Results similar to these have been shown before for brood and colony V. destructor levels (Delaplane et al. 2015, 2021), joining many other papers reporting improved colony fitness measures with increasing polyandry (Tarpy 2003; Graham et al. 2006; Mattila and Seeley 2007; Seeley and Tarpy 2007; Girard et al. 2011; Desai and Currie 2015; Eckholm et al. 2015; Soper et al. 2021). However, the present design and inclusion of an experiment on worker task distribution offer insights into how polyandry evolved from incipient lows to averages as high as 40–44 as seen in modern Apini (Tarpy et al. 2004).
We want to make clear two ways in which we constrained random genetic variation in this study: experimental queens were all supersisters of one wild type non-selected mother, and the drones they were mated to were sons of 10 different wild type non-selected queens/colonies. These measures were taken to focus on the effects of polyandry in a narrow sense. If our constraint on maternal variation exaggerates polyandry effects, our constraint on drone source colonies understates it. Clearly, work like ours should be replicated under more natural ranges and distributions of population genetic variation, but the approach we take here reduces noise and affirms the agency of increasing mating number as a driver of colony-level changes in a vector predictable for social evolution.
Brood area
Our results for brood area support our hypothesis #1: colony gains in cooperative brood care, an ancestral task foundational to eusociality, track the geometric increase and plateau of within-colony genetic diversity consistent with the PAC model. The curves for brood area (Fig. 2) closely match the curve for predicted changes in within-colony genetic diversity by mating number as constrained by haplo-diploid inheritance (Fig. 1) (Palmer and Oldroyd 2000).
Genes contributing to aggregate brood production include those responsible for the egg-yolk protein vitellogenin and its effects on queen fecundity and worker nursing (Kent et al. 2011; Cardoso‐Júnior et al. 2021), the major royal jelly proteins (Consortium 2006), nest temperature regulation (Jones et al. 2004), and worker foraging preferences for pollen or nectar (Hunt et al. 2007), to name a few. But more to the point, theory (West-Eberhard 1996; Linksvayer and Wade 2005) and experimental results (Amdam et al. 2006; Cardoso‐Júnior et al. 2021) both support that ancient gene networks associated with solitary maternal behavior were coopted into pathways governing sibling care by sterile workers – a defining tenet of eusociality. As such, we can expect cooperative brood care to be present and sensitive to patriline specialisms and efficiency gains acquired at incipient stages of polyandry evolution. The shape of the curves in Fig. 2 suggests that these gains mirrored the geometric increase in allele capture shown in Fig. 1 and occurred in a gradualistic manner up to me∼20. However, we note the decline in brood area at mo = 32 which was universal except for the Oct timepoint. One explanation for this is the possibility of conflict among increasing numbers of specialists, especially if those individuals inherit different action thresholds for focal tasks (Calderone and Page 1991). Evidence for negative interactions among patrilines has been shown for at least one indicator of colony fitness, seasonal weight gain (Oldroyd et al. 1992). Specialist conflict may pose a constraint on upper queen mating numbers alongside others such as bird predation and risks of sexually transmitted diseases (Schlüns et al. 2005; De Miranda and Fries 2008; Roberts et al. 2015).
To put the present results in perspective we must consider that brood production also increases at polyandry levels higher than the me∼10 asymptote and therefore outside the predictions of the PAC model. Delaplane et al. (2015) showed higher brood rearing efficiency (cm2 brood per 100 bees) in colonies whose queens were inseminated at mo = 30 or = 60 compared to queens inseminated at mo = 15, and Delaplane et al. (2021) showed that 3-day brood survival was higher in colonies whose queens were inseminated at mo = 54 compared to queens inseminated at mo = 9. Clearly, many gene sets or individual specialist traits contribute to aggregate brood production, some of which are responsive to mating levels in the hyperpolyandrous zone.
Mite counts
In contrast to results for brood area, colony populations of the non-natural parasite Varroa destructor declined with increasing mating numbers in an exclusively linear manner (Fig. 3), supporting our hypothesis #2: colony fitness gains in a derived character are not constrained by the rules of hymenopteran inheritance and are free to increase linearly in compliance with the GC model.
Increasing polyandry alone did not reduce colony mite populations, as V. destructor numbers increased every month from June to October in a pattern typical on its non-natural host Apis mellifera (Martin 1998); however, within each timepoint increasing queen mating number was associated with significantly slower rates of mite population growth.
We are interested primarily, however, in the fact that colony fitness gains continued in a linear fashion up to the highest mating level tested, mo = 32. Other studies have shown Varroa mitigation at mating levels of mo = 30, 60 (Delaplane et al. 2015), or 54 (Delaplane et al. 2021). In other words, the linear trend for improved colony resistance to V. destructor at hyperpolyandrous mating levels is robust with no upper limit yet detected. This pattern begs for an alternate explanation because it is incongruent with the PAC model that predicts diminishing returns for mating numbers above me = 10.
Fuchs and Moritz (1999) proposed that colony fitness gains in the hyperpolyandry zone accrue not from additive population allele capture but rather from the random acquisition of unique genotype compositions. While the pool of population alleles is finite and their capture constrained in Hymenoptera in the manner shown in Fig. 1, the number of unique genotype compositions is bound by no upper limit. Although these infinitely unique genotypes include combinations that favor common ancestral tasks (as noted above for brood production), it seems likely that changes in ancestral states are weighted toward polyandry changes early in social evolution and below the me ~ 10 asymptote. In contrast, it is in the derived upper reaches of hyperpolyandry that we find the kinds of unique allele compositions that mitigate against rare, new, or idiosyncratic cliff-edge ecologic catastrophes (Mountford 1968; Boyce and Perrins 1987) such as the lethal non-natural parasite V. destructor (Delaplane et al. 2021). The negative linear relationship we show here between colony mite levels and queen mating number mirrors the prediction of Fuchs and Moritz (1999) that optimal genotype compositions continue increasing infinitely above the me ~ 10 asymptote; indeed it is in this zone that their model predicts the highest fitness returns.
Our results are also supported from the perspective of epidemiological theory on disease and parasite virulence evolution. Ball (1985) and subsequent authors (Dwyer et al. 1997) have shown that reduced infectious disease burdens are predicted in host populations with highly heterogeneous individuals compared to populations with homogenous individuals even if average susceptibility to infection is the same in both populations. Figure 1B in Dwyer et al. (1997) in particular illustrates the importance of increasing host heterogeneity at reducing rates of disease transmission as environmental pathogen density increases. This hints at another mechanism selecting for hyperpolyandry in eusocial evolution. As colonies increased in complexity, task efficiency, and population size, hyperpolyandry would have provided levels of population heterogeneity sufficient to offset the simultaneous increase in pathogen transmissibility afforded by increasing host density in finite nest cavities. Host heterogeneity decreases opportunity for a parasite to specialize into one dominant virulent genotype (Brosi et al. 2017). In the context of the economically important western honey bee Apis mellifera and its non-natural parasite Varroa destructor, whenever improved colony survival has been detected whether in natural populations or as a result of controlled breeding, the resistance mechanisms are numerous, expressing phenotypic variants across behaviors, individual immunities, population dynamics, and interactions with associated pathogens. Poignantly, they share no common molecular pathways (Mondet et al. 2020). One cannot escape the possibility that any variation at all will help reduce total parasite burden in large host populations.
Worker task distributions
Whether genetic novelty accumulates through population allele capture (hypothesis #1) or unique genotypes (hypothesis #2), its effects should collapse into observable differences in worker task distributions. We found that same-aged siblings of a mother mated at mo = 16 performed more tasks (Fig. 4) and more types of tasks (Fig. 5) per observed period than an identically aged cohort from a mother mated at mo = 32, supporting our hypothesis #3: changes in polyandry state will be reflected in detectable changes in worker task distributions. Specialisms based on paternity have been shown for many life-critical common tasks including guarding, undertaking, pollen collecting, nectar foraging, scouting, regulating nest temperature, and nursing larvae (Calderone and Page 1991; Chapman et al. 2007; Jones et al. 2007). Although no formal definition of a specialist has been put forward for Apis mellifera, it is generally understood to be an individual’s bias in favor of a focal task, measured either by its exclusivity for that task or the time spent performing it (see, for example, Nakamura and Seeley (2006)). By extension, a specialist will perform fewer kinds of tasks in her lifetime and, quite possibly, fewer tasks altogether owing to group efficiencies. By the measures taken here, siblings from the mo = 32 mother are expressing higher rates of specialism compared to workers from the mo = 16 mother: with half the patrilines to recruit from, individuals in the mo = 16 cohort are pressed into performing more types of tasks to meet colony needs. As the mo = 16 cohort represents the fringe of the me = 10 asymptote while the mo = 32 cohort is solidly in the hyperpolyandry zone (3X higher than the me = 10 break point; Fig. 1), these results support the longstanding assumption that benefits from polyandry are linked to increased worker specialisms and task efficiencies (Gove et al. 2009). What is new in our present results is a demonstration that this mechanism holds across the continuum from ancestral states of incipient polyandry to derived states of hyperpolyandry.
Conclusions
We conclude that (1) ancestral colony-level phenotypes fundamental to eusociality are responsive to mating level changes at or below the me ~ 10 ceiling imposed by rules of hymenopteran inheritance, whereas (2) more derived specialized colony phenotypes such as resistance to a non-natural parasite are responsive to mating levels unconstrained by this ceiling and continue improving with increasing me. By either scenario, (3) the mechanism for increasing colony level fitness is an increase in worker task specialisms and task efficiency. The population allele capture (PAC) and genotype composition (GC) models thus together explain the whole range of mating numbers seen in the complex social bees of Apini.
We postulate that ancestral traits foundational to eusociality were responsive to early forays into polyandry, prompting mating numbers to increase in a classically gradualistic manner to the ceiling on population allele capture imposed by haplo-diploid inheritance. Ecologic hazards and increasing risks of parasite/pathogen transmission with increasing nest population densities were, in turn, the drivers selecting for even higher mating numbers. Extremely promiscuous queens captured rare, highly potent alleles and increased colony population heterogeneity, enabling colonies to resist rising risks of parasite and pathogen transmission and overcome ecologic stressors that were novel or idiosyncratic.
Data availability
The dataset generated and analyzed during the current study are available in the Dryad repository, https://doi.org/10.5061/dryad.fqz612k0x.
References
Amdam GV, Csondes A, Fondrk MK, Page RE (2006) Complex social behaviour derived from maternal reproductive traits. Nature 439:76
Bakhtin Y, Katsnelson MI, Wolf YI, Koonin EV (2021) Evolution in the weak-mutation limit: stasis periods punctuated by fast transitions between saddle points on the fitness landscape. Proc Natl Acad Sci 118(4):e2015665118
Ball F (1985) Deterministic and stochastic epidemics with several kinds of susceptibles. Adv Appl Probab 17:1–22
Barth MB, Moritz RFA, Kraus FB (2014) The evolution of extreme polyandry in social insects: insights from army ants. PLoS ONE 9:e105621
Bartlett LJ, Bruckner S, Delaney DA, Williams GR, Delaplane KS (2022) A computational approach to tracking age-based task frequency distributions of Apis mellifera worker cohorts. J Apic Res 61:147–150
Boyce MS, Perrins C (1987) Optimizing great tit clutch size in a fluctuating environment. Ecology 68:142–153
Brosi BJ, Delaplane KS, Boots M, de Roode JC (2017) Ecological and evolutionary approaches to managing honeybee disease. Nat Ecol Evol 1:1250
Calderone NW, Page RE (1991) Evolutionary genetics of division of labor in colonies of the honey bee (Apis mellifera). Am Nat 138:69–92
Cardoso-Júnior CAM, Oldroyd BP, Ronai I (2021) Vitellogenin expression in the ovaries of adult honeybee workers provides insights into the evolution of reproductive and social traits. Insect Mol Biol 30:277–286
Chapman NC, Oldroyd BP, Hughes WO (2007) Differential responses of honeybee (Apis mellifera) patrilines to changes in stimuli for the generalist tasks of nursing and foraging. Behav Ecol Sociobiol 61:1185–1194
Cobey SW, Tarpy DR, Woyke J (2013) Standard methods for instrumental insemination of Apis mellifera queens. J Apic Res 52:1–18
Consortium HGS (2006) Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443:931
Crozier RH, Fjerdingstad EJ (2001) Polyandry in social Hymenoptera—disunity in diversity? Ann Zool Fenn 38:267–285
De Miranda J, Fries I (2008) Venereal and vertical transmission of deformed wing virus in honeybees (Apis mellifera L.). J Invertebr Pathol 98:184–189
Delaplane KS, van der Steen J, Guzman-Novoa E (2013) Standard methods for estimating strength parameters of Apis mellifera colonies. J Apic Res 52:1–12
Delaplane KS, Pietravalle S, Brown MA, Budge GE (2015) Honey bee colonies headed by hyperpolyandrous queens have improved brood rearing efficiency and lower infestation rates of parasitic Varroa mites. PLoS ONE 10:e0142985
Delaplane KS, Given JK, Menz J, Delaney DA (2021) Colony fitness increases in the honey bee at queen mating frequencies higher than genetic diversity asymptote. Behav Ecol Sociobiol 75:1–12
Desai SD, Currie RW (2015) Genetic diversity within honey bee colonies affects pathogen load and relative virus levels in honey bees, Apis mellifera L. Behav Ecol Sociobiol 69:1527–1541
Dietemann V, Nazzi F, Martin S, Anderson D, Locke B, Delaplane K, Wauquiez Q, Tannahill C, Frey E, Ziegelmann B, Rosenkranz P, Ellis J (2013) Standard methods for Varroa research. J Apic Res 52:1–54
Dwyer G, Elkinton JS, Buonaccorsi JP (1997) Host heterogeneity in susceptibility and disease dynamics: tests of a mathematical model. Am Nat 150:685–707
Eckholm BJ, Anderson KE, Weiss M, DeGrandi-Hoffman G (2011) Intracolonial genetic diversity in honeybee (Apis mellifera) colonies increases pollen foraging efficiency. Behav Ecol Sociobiol 65:1037–1044
Eckholm BJ, Huang MH, Anderson KE, Mott BM, DeGrandi-Hoffman G (2015) Honey bee (Apis mellifera) intracolonial genetic diversity influences worker nutritional status. Apidologie 46:150–163
Fuchs S, Moritz R (1999) Evolution of extreme polyandry in the honeybee Apis mellifera L. Behav Ecol Sociobiol 45:269–275
Girard M, Mattila H, Seeley T (2011) Recruitment-dance signals draw larger audiences when honey bee colonies have multiple patrilines. Insectes Soc 58:77–86
Gove R, Hayworth M, Chhetri M, Rueppell O (2009) Division of labour and social insect colony performance in relation to task and mating number under two alternative response threshold models. Insectes Soc 56:319–331
Graham S, Myerscough M, Jones J, Oldroyd B (2006) Modelling the role of intracolonial genetic diversity on regulation of brood temperature in honey bee (Apis mellifera L.) colonies. Insectes Soc 53:226–232
Hunt GJ, Amdam GV, Schlipalius D, Emore C, Sardesai N, Williams CE, Rueppell O, Guzmán-Novoa E, Arechavaleta-Velasco M, Chandra S (2007) Behavioral genomics of honeybee foraging and nest defense. Naturwissenschaften 94:247–267
Jones JC, Myerscough MR, Graham S, Oldroyd BP (2004) Honey bee nest thermoregulation: diversity promotes stability. Science 305:402–404
Jones JC, Nanork P, Oldroyd BP (2007) The role of genetic diversity in nest cooling in a wild honey bee, Apis florea. J Comp Physiol A 193:159–165
Katsnelson MI, Wolf YI, Koonin EV (2019) On the feasibility of saltational evolution. Proc Natl Acad Sci 116:21068–21075
Kent CF, Issa A, Bunting AC, Zayed A (2011) Adaptive evolution of a key gene affecting queen and worker traits in the honey bee, Apis mellifera. Mol Ecol 20:5226–5235
Kraus F, Neumann P, Van Praagh J, Moritz R (2004) Sperm limitation and the evolution of extreme polyandry in honeybees (Apis mellifera L.). Behav Ecol Sociobiol 55:494–501
Kraus F, Neumann P, Moritz R (2005) Genetic variance of mating frequency in the honeybee (Apis mellifera L.). Insectes Soc 52:1–5
Kronauer DJ, Schöning C, Pedersen JS, Boomsma JJ, Gadau J (2004) Extreme queen-mating frequency and colony fission in African army ants. Mol Ecol 13:2381–2388
Linksvayer TA, Wade MJ (2005) The evolutionary origin and elaboration of sociality in the aculeate Hymenoptera: maternal effects, sib-social effects, and heterochrony. Q Rev Biol 80:317–336
Mackensen O (1947) Effect of carbon dioxide on initial oviposition of artificially inseminated and virgin queen bees. J Econ Entomol 40:344–349
Martin S (1998) A population model for the ectoparasitic mite Varroa jacobsoni in honey bee (Apis mellifera) colonies. Ecol Modell 109:267–281
Mattila HR, Seeley TD (2007) Genetic diversity in honey bee colonies enhances productivity and fitness. Science 317:362–364
Mattila HR, Seeley TD (2010) Promiscuous honeybee queens generate colonies with a critical minority of waggle-dancing foragers. Behav Ecol Sociobiol 64:875–889
Mattila HR, Seeley TD (2011) Does a polyandrous honeybee queen improve through patriline diversity the activity of her colony’s scouting foragers? Behav Ecol Sociobiol 65:799–811
Mattila HR, Seeley TD (2014) Extreme polyandry improves a honey bee colony’s ability to track dynamic foraging opportunities via greater activity of inspecting bees. Apidologie 45:347–363
Mattila HR, Burke KM, Seeley TD (2008) Genetic diversity within honeybee colonies increases signal production by waggle-dancing foragers. Proc R Soc B: Biol Sci 275:809–816
Mondet F, Beaurepaire A, McAfee A, Locke B, Alaux C, Blanchard S, Danka B, Le Conte Y (2020) Honey bee survival mechanisms against the parasite Varroa destructor: a systematic review of phenotypic and genomic research efforts. Int J Parasitol 50:433–447
Mountford M (1968) The significance of litter-size. J Anim Ecol 37:363–367
Nakamura J, Seeley TD (2006) The functional organization of resin work in honeybee colonies. Behav Ecol Sociobiol 60:339–349
Nonacs P, Kapheim K (2007) Social heterosis and the maintenance of genetic diversity. J Evol Biol 20:2253–2265
Oldroyd BP, Rinderer TE, Harbo JR, Buco SM (1992) Effects of intracolonial genetic diversity on honey bee (Hymenoptera: Apidae) colony performance. Ann Entomol Soc Am 85:335–343
Oldroyd BP, Smolenski AJ, Cornuet J-M, Wongsiri S, Estoup A, Rinderer TE, Crozier RH (1995) Levels of polyandry and intracolonial genetic relationships in Apis florea. Behav Ecol Sociobiol 37:329–335
Page RE (1980) The evolution of multiple mating behavior by honey bee queens (Apis mellifera L.). Genetics 96:263–273
Palmer KA, Oldroyd BP (2000) Evolution of multiple mating in the genus Apis. Apidologie 31:235–248
Payne AN, Rangel J (2018) The effect of queen insemination volume on the growth of newly established honey bee (Apis mellifera) colonies. Apidologie 49:594–605
Ratnieks FL, Helanterä H (2009) The evolution of extreme altruism and inequality in insect societies. Philos Trans R Soc London B: Biol Sci 364:3169–3179
Roberts K, Evison S, Baer B, Hughes W (2015) The cost of promiscuity: Sexual transmission of Nosema microsporidian parasites in polyandrous honey bees. Sci Rep 5:10982
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103:S96–S119
Schlüns H, Moritz RF, Neumann P, Kryger P, Koeniger G (2005) Multiple nuptial flights, sperm transfer and the evolution of extreme polyandry in honeybee queens. Anim Behav 70:125–131
Seeley TD, Tarpy DR (2007) Queen promiscuity lowers disease within honeybee colonies. Proc R Soc B: Biol Sci 274:67–72
Seeley TD, Visscher PK (1985) Survival of honeybees in cold climates: the critical timing of colony growth and reproduction. Ecol Entomol 10:81–88
Simone-Finstrom M, Tarpy DR (2018) Honey bee queens do not count mates to assess their mating success. J Insect Behav 31:200–209
Soper D, Ekroth A, Martins M (2021) Direct evidence for increased disease resistance in polyandrous broods exists only in eusocial Hymenoptera. BMC Ecology and Evolution 21:1–10
Starr CK (1985) Sperm competition, kinship, and sociality in the aculeate Hymenoptera. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic Press, Orlando, pp 427–464
Tarpy DR (2003) Genetic diversity within honeybee colonies prevents severe infections and promotes colony growth. Proc R Soc B: Biol Sci 270:99
Tarpy DR, Page RE (2000) No behavioral control over mating frequency in queen honey bees (Apis mellifera L.): implications for the evolution of extreme polyandry. Am Nat 155:820–827
Tarpy DR, Seeley TD (2006) Lower disease infections in honeybee (Apis mellifera) colonies headed by polyandrous vs monandrous queens. Naturwissenschaften 93:195–199
Tarpy D, Nielsen R, Nielsen D (2004) A scientific note on the revised estimates of effective paternity frequency in Apis. Insectes Soc 51:203–204
Van Dooremalen C, Gerritsen L, Cornelissen B, van der Steen JJ, van Langevelde F, Blacquiere T (2012) Winter survival of individual honey bees and honey bee colonies depends on level of Varroa destructor infestation. PLoS ONE 7:e36285
West-Eberhard MJ (1996) Wasp societies as microcosms for the study of development and evolution. In: Turillazzi S, West-Eberhard MJ (eds) Natural history and evolution of paper wasps. Oxford Universitiy Press, Oxford, pp 290–317
Withrow JM, Tarpy DR (2018) Cryptic “royal” subfamilies in honey bee (Apis mellifera) colonies. PLoS ONE 13:e0199124
Yasui Y (2001) Female multiple mating as a genetic bet-hedging strategy when mate choice criteria are unreliable. Ecol Res 16:605–616
Acknowledgements
We gratefully acknowledge the field assistance of Jennifer Berry, Jack Garrison, and Ian Rafael Collins. Statistical assistance was provided by Kim Love.
Funding
This research was supported by the Myron Schaer Memorial Endowment at the University of Georgia, the Georgia Beekeepers Association, and the Foundation for Food and Agriculture Research, award number 19EPP244591UGA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Toth
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Delaplane, K., Hagan, K., Vogel, K. et al. Mechanisms for polyandry evolution in a complex social bee. Behav Ecol Sociobiol 78, 39 (2024). https://doi.org/10.1007/s00265-024-03450-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03450-x