Abstract
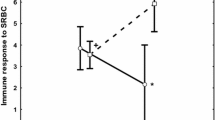
The parasite-mediated sexual selection hypothesis predicts that color expression in color patches of animals can honestly reflect male quality in terms of resistance to parasites. Sceloporine lizards have structural-based blue color patches that can act as intraspecific signals and may thus reflect immunocompetence. However, both color patch expression and intensity of parasitic infections in lizards can vary across seasons. In consequence, we might expect that coloration would honestly reflect immunocompetence to resist parasites only during the mating season. We sampled males of Sceloporus torquatus in central Mexico in spring, summer, and autumn and quantified the reflectance of two structural-based color patches (throat and venter), abundance of two categories of parasites (mites and hemoparasites), and lizards’ local inflammatory response to a mitogen (IRM) as a measure of immunocompetence. We examined whether (i) the coloration of lizards changed across seasons in the population, (ii) there is a relationship between coloration and parasite load and/or IRM, and (iii) the latter relationships remained consistent across seasons. Our study shows that color expression seasonally varied; the structural-based coloration of the two patches was significantly more intense in summer, before the mating season. Furthermore, the throat color was more intense in those males with lower parasite load and higher IRM. However, season had no effect on these relationships, suggesting that color expression in the males of S. torquatus can consistently reflect some components of their immunocompetence throughout the year, supporting the honesty of the structural-based coloration in this species.
Significance statement
In this study, we aimed to investigate the seasonal variation in structural coloration of a lizard species and its potential relationship with male quality in Torquate lizards (Sceloporus torquatus). Our results revealed distinct seasonal differences in color expression, and furthermore, we found that males displaying more intense blue coloration exhibited lower parasite loads and stronger immune responses. These findings contribute to our understanding of two key aspects: (i) the potential role of structural coloration as an honest signal in organisms of this nature, and (ii) the significance of considering sampling times in organisms with structural coloration, as it can vary throughout the year.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Assis BA, Jarrett BJ, Koscky G, Langkilde T, Avery JD (2020) Plastic sexual ornaments: assessing temperature effects on color metrics in a color-changing reptile. PLOS ONE 15:e0233221
Baeckens S, Van Damme R (2018) Immunocompetence and parasite infestation in a melanistic and normally-coloured population of the lacertid lizard, Podarcis siculus. Amphibia-Reptilia 39:471–478
Bajer K, Molnár O, Török J, Herczeg G (2011) Ultraviolet nuptial colour determines fight success in male European green lizards (Lacerta viridis). Biol Lett 7:866–868
Barton K (2013) MuMIn: Multi-model inference. R package, version 1.40.0, https://cran.r-project.org/web/packages/MuMIn/index.html
Bedrick EJ, Tsai CL (1994) Model selection for multivariate regression in small samples. Biometrics 50:226–231
Bohórquez-Alonso ML, Molina-Borja M (2014) Reflectance of sexually dichromatic UV-blue patches varies during the breeding season and between two subspecies of Gallotia galloti (Squamata: Lacertidae). Biol J Linn Soc 113:556–569
Borgia G, Collias K (1990) Parasites and bright male plumage in the satin bowerbird (Ptilonorhynchus violaceus). Am Zool 30:279–285
Braun C, Michiels NK, Siebeck UE, Sprenger D (2014) Signaling function of long wavelength colors during male agonistics male-male in interaction in the wrasse Coris julis. Mar Ecol Prog Ser 504:277–228
Burtt EH (1979) The behavioral significance of color. Garland Press, New York
Calisi RM, Hews DK (2007) Steroid correlates of multiple color traits in the spiny lizard, Sceloporus pyrocephalus. J Comp Physiol B 177:641–654
Cooper WE, Burns N (1987) Social significance of ventrolateral coloration in the fence lizard, Sceloporus undulatus. Anim Behav 35:526–532
Courty Y, Dufaure JP (1980) Levels of testosterone, dihydrotesterone, and androstenedione in the plasma and testis of a lizard (Lacerta vivipara Jacquin) during the annual cycle. Gen Comp Endocrinol 42:325–333
Cox R, John-Alder HB (2007) Increased mite parasitism as a cost of testosterone in male striped plateau lizards Sceloporus virgatus. Funct Ecol 21:327–334
Cox RM, Skelly SL, Leo A, John-Alder HB (2005) Testosterone regulates sexually dimorphic coloration in the Eastern Fence Lizard, Sceloporus undulatus. Copeia 2005:597–608
Cox RM, Zilberman V, John-Alder HB (2008) Testosterone stimulates the expression of a social color signal in Yarrow’s Spiny Lizard, Sceloporus jarrovii. J Exp Zool A 309:505–514
Delhey K, Burger C, Fiedler W, Peters A (2010) Seasonal changes in colour: a comparison of structural, melanin and carotenoid based plumage colours. PLoS ONE 5:e11582
Escudero PC, Minoli I, Gonzalez-Marin MA, Morando M, Avila LJ (2016) Melanism and ontogeny: a case study in lizards of the Liolaemus fitzingerii group (Squamata: Liolaemini). Can J Zool 94:199–206
Feria-Ortiz M, Nieto-Montes de Oca A, Salgado-Ugarte IH (2001) Diet and reproductive biology of the viviparous lizard Sceloporus torquatus torquatus (Squamata: Phrynosomatidae). J Herpetol 35:104–112
Folstad I, Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. Am Nat 139:603–622
French SS, DeNardo DF, Moore MC (2007) Trade-offs between the reproductive and immune systems: facultative responses to resources or obligate responses to reproduction? Am Nat 170:79–89
González-Morales JC, Rivera-Rea J, Moreno-Rueda G, Bastiaans E, Díaz-Albiter H, Díaz de la Vega-Pérez AH, Bautista-Ortega A, Fajardo V (2021) To be small and dark is advantageous for gaining heat in mezquite lizards, Sceloporus grammicus (Squamata: Phrynosomatidae). Biol J Linn Soc 132:93–103
Griggio M, Serra L, Licheri D, Campomori C, Pilastro A (2009) Moult speed affects structural feather ornaments in the blue tit. J Evol Biol 22:782–792
Griggio M, Zanollo V, Hoi H (2010) UV plumage color is an honest signal of quality in male budgerigars. Ecol Res 25:77–82
Guzmán-Cornejo C, García-Prieto L, Zúñiga-Vega J (2018) First quantitative data on the ectoparasites mites of Sceloporus torquatus (Squamata) from the Ecological Reserve of Pedregal de San Angel in Central Mexico. Acarologia 55:868–874
Halliday WD, Paterson JE, Patterson LD, Cooke SJ, Blouin-Demers G (2014) Testosterone, body size, and sexual signals predict parasite load in Yarrow’s Spiny Lizards (Sceloporus jarrovii). Can J Zool 92:1075–1084
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Hartig F (2021) DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. R Package Version 0.4.4, https://cran.r-project.org/web/packages/DHARMa/index.html
Huyghe K, Van Oystaeyen A, Pasmans F, Tadić Z, Vanhooydonck B, Van Damme R (2010) Seasonal changes in parasite load and a cellular immune response in a color polymorphic lizard. Oecologia 163:867–874
i de Lanuza GP, Carazo P, Font E (2014) Colours of quality: structural (but not pigment) coloration informs about male quality in a polychromatic lizard. Anim Behav 90:73–81
INEGI (2009) Instituto Nacional de Estadística, Geografía e Informática, prontuario de información geográfica municipal de los Estados Unidos Mexicanos. Geostatisticalkey:15098 https://www.inegi.org.mx/contenidos/app/mexicocifras/datos_geograficos/15/15098.pdf
Jackman S (2012) pscl: Classes and methods for R developed in the political science computational laboratory, Stanford University, R package version 1.04.1, http://pscl.stanford.edu/
Klukowski M, Nelson CE (2001) Ectoparasite loads in free-ranging northern fence lizards, Sceloporus undulatus hyacinthinus: effects of testosterone and sex. Behav Ecol Sociobiol 49:289–295
Langkilde T, Boronow KE (2012) Hot boys are blue: temperature-dependent color change in male eastern fence lizards. J Herpetol 46:461–465
Liner EA, Olson RE (1973) Adults of the lizard Sceloporus torquatus binocularis Dunn. Herpetologica 29(1):53–55
López P, Gabirot M, Martín J (2009) Immune challenge affects sexual coloration of male Iberian wall lizards. J Exp Zool A 311:96–104
Martín J, Amo L, López P (2008) Parasites and health affect multiple sexual signals in male common wall lizards, Podarcis muralis. Sci Nat 95:293–300
McCullough EL, Miller CW, Emlen DJ (2016) Why sexually selected weapons are not ornaments. Trend Ecol Evol 31:742–751
McKinney RB, Marion KR (1985) Plasma androgens and their association with the reproductive cycle of the male fence lizard, Sceloporus undulatus. Comp Biochem Physiol A 82:515–519
Megía-Palma R, Barrientos R, Gallardo M, Martínez J, Merino S (2021) Brighter is darker: the Hamilton–Zuk hypothesis revisited in lizards. Biol J Linn Soc 134:461–473
Megía-Palma R, Jorge A, Reguera S (2018a) Raman spectroscopy reveals the presence of both eumelanin and pheomelanin in the skin of lacertids. J Herpetol 52:67–73
Megía-Palma R, Martínez J, Merino S (2013) Phylogenetic analysis based on 18S rRNA gene sequences of Schellackia parasites (Apicomplexa: Lankesterellidae) reveals their close relationship to the genus Eimeria. Parasitology 140:1149–1157
Megía-Palma R, Martínez J, Merino S (2016a) Structural- and carotenoid-based throat colour patches in males of Lacerta schreiberi reflect different parasitic diseases. Behav Ecol Sociobiol 70:2017–2025
Megía-Palma R, Martínez J, Merino S (2016b) A structural colour ornament correlates positively with parasite load and body condition in an insular lizard species. Sci Nat 103:7–8
Megía-Palma R, Martínez J, Merino S (2018c) Manipulation of parasite load induces significant changes in the structural-based throat color of male Iberian green lizards. Curr Zool 64:293–302
Megía-Palma R, Merino S, Barrientos R (2022) Longitudinal effects of habitat quality, body condition, and parasites on colour patches of a multiornamented lizard. Behav Ecol Sociobiol 76:73
Megía-Palma R, Paranje D, Reguera S, Martínez J, Cooper RD, Blaimont P, Merino S, Sinervo B (2018b) Multiple color patches and parasites in Sceloporus occidentalis: differential relationships by sex and infection. Curr Zool 64:703–711
Megía-Palma R, Paranjpe D, Cooper RD, Blaimont P, Sinervo B (2023, 2022) Natural parasites in conjunction with behavioral and color traits explain male agonistic behaviors in a lizard. Curr Zool:zoac095
Merino S, Potti J (1995) High prevalence of hematozoa in nestlings of a passerine species, the pied flycatcher, Ficedula hypoleuca. Auk 112:1041–1043
Mészáros B, Jordán L, Bajer K, Martín J, Török J, Molnár O (2019) Relationship between oxidative stress and sexual coloration of lizards depends on thermal habitat. Sci Nat 106:55
Molnár O, Bajer J, Mészáros B, Török J, Herczeg G (2013) Negative correlation between nuptial throat colour and blood parasite load in male European green lizards supports the Hamilton-Zuk hypothesis. Sci Nat 100:551–558
Montgomerie R (2006) Quantifying colors. In: Hill GE, McGraw KJ (eds) bird coloration, Mechanisms and measurements, vol 1. Harvard University Press, Cambridge, MA, pp 90–147
Montgomerie R (2009) CLR, version 1.1. Queen’s University, Kingston, Canada http://post.queensu.ca/~mont/color/analyze.html
Moore M (1986) Elevated testosterone levels during nonbreeding-season territoriality in a fall-breeding lizard, Sceloporus jarrovi. J Comp Physiol A 158:159–163
Moore MC (1988) Testosterone control of territorial behavior: tonic-release implants fully restore seasonal and short-term aggressive responses in free-living castrated lizards. Gen Comp Endocrinol 70:450–459
Olsson M, Stuart-Fox D, Ballen C (2013) Genetics and evolution of colour patterns in reptiles. Semin Cell Dev Biol 24:529–541
Örnborg J, Andersson S, Griffith S, Shelldon B (2002) Seasonal change in an ultraviolet structural color signal in blue tits, Parus caeruleus. Biol J Linn Soc 76:237–245
Orton RW, Kinsey CT, McBrayer LD (2019) Mite load predicts the quality of sexual color and locomotor performance in sexually dichromatic lizard. Ecol Evol 10:3152–3163
Plasman M, Reynoso VH, Nicolás L, Torres R (2015) Multiple colour traits signal performance and immune response in the Dickerson’s collared lizard Crotaphytus dickersonae. Behav Ecol Sociobiol 69:765–775
Quinn VS, Hews DK (2003) Positive relationship between abdominal coloration and dermal melanin density in phrynosomatid lizards. Copeia 2003:858–864
R Core Team (2020) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria http://www.R-project.org
Rand MS (1992) Hormonal control of polymorphic and sexually dimorphic coloration in the lizard Sceloporus undulatus erythrocheilus. Gen Comp Endocrinol 88(3):461–468
Rémy A, Grégoire A, Perret P, Doutrelant C (2010) Mediating male-male interactions: the role of the UV blue crest coloration in blue tits. Behav Eco Sociobiol 64:1839–1847
Rivera-Rea J, González-Morales JC, Fajardo V, Megía-Palma R, Bastiaans E, Manjarrez J (2022) Phenological variation in parasite load and inflammatory response in a lizard with an asynchronous reproductive cycle. Sci Nat 109:34
Roberts ML, Buchanan KL, Evans MR (2004) Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim Behav 68:227–239
Robinson CD, Gifford ME (2019) Intraseasonal changes of patch color in prairie lizards (Sceloporus consobrinus). Herpetologica 75:79–84
Rogier É, Landau I (1975) Description de Schellackia golvani n. sp. (Lankesterellidae), parasite de Lézards de Guadeloupe. Bull Mus Nat Hist Nat 284:91–97
Salvador A, Veiga JP, Martin J, Lopez P, Abelenda M, Puertac M (1996) The cost of producing a sexual signal: testosterone increases the susceptibility of male lizards to ectoparasitic infestation. Behav Ecol 7:145–150
Salvador A, Veiga P (2008) A permanent signal related to male pairing success and survival in the lizards Psammodromus algirus. Amphibia-Reptilia 29:117–120
Schall JJ (1986) Prevalence and virulence of a haemogregarine parasite of the Aruban whiptail lizard, Cnemidophorus arubensis. J Herpetol 20:318–324
Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ (2005) Restitution of mass–size residuals: validating body condition indices. Ecology 86:155–163
Shawkey MD, Estes AM, Siefferman LM, Hill GE (2003) Nanostructure predicts intraspecific variation in ultraviolet-blue plumage color. Proc R Soc Lond B 270:1455–1460
Sheldahl LA, Martins EP (2000) The territorial behavior of the western fence lizard, Sceloporus occidentalis. Herpetologica 56:469–479
Siefferman L, Hill GE (2003) Structural and melanin coloration indicate parental effort and reproductive success in male eastern bluebirds. Behav Ecol 14:855–861
Smith HM (1936) The lizards of the torquatus group of the genus Sceloporus Wiegmann, 1828. Univ Kans Sci Bull 24:539–693
Smits JE, Bortolotti GR, Tella JL (1999) Simplifying the phytohaemagglutinin skin-testing technique in studies of avian immunocompetence. Funct Ecol 13:567–572
Stephenson BP, Ihász N, Byrd DC, Swierk J, Swierk L (2017) Temperature-dependent colour change is a function of sex and directionality of temperature shift in the eastern fence lizard (Sceloporus undulatus). Biol J Linn Soc 120:396–409
Svensson E, Sinervo B, Comendant T (2001) Density-dependent competition and selection on immune function in genetic lizard morphs. P Natl Acad Sci USA 98:12561–12565
Umbers KD (2013) On the perception, production and function of blue coloration in animals. J Zool 289:229–242
Václav R, Prokop P, Fekiač V (2007) Expression of breeding coloration in European green lizards (Lacerta viridis): variation with morphology and tick infestation. Can J Zool 85:1199–1206
Zahavi A (1975) Mate selection—a selection for a handicap. J Theor Biol 53:205–214
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Acknowledgements
Many thanks go to Gabriel Suárez and Luis Macotela for field assistance and Mercedes Pichardo for their help and technical support. Special thanks to the anonymous reviewers for their thoughtful and constructive comments that improved the manuscript. We are grateful to the Consejo Nacional de Ciencia y Tecnología (PhD degree scholarship JR-R and JCG-M) and the Secretaría del Medio Ambiente y Recursos Naturales for the permits to collect animals (SGPA/DGVS/02407/15).
Funding
This work was funded by Consejo Nacional de Ciencia y Tecnología (PhD degree scholarship JR-R).
Author information
Authors and Affiliations
Contributions
JM and JR-R formulated the idea and designed the experiments. JR-R and JCG-M performed the experiments. JR-R and RM-P analyzed the data. JCG-M and EQ conducted fieldwork. JR-R, EB, and JCG-M wrote the manuscript. EB, EQ, and JM provided editorial advice.
Corresponding author
Ethics declarations
Ethical approval
All experimental procedures were carried out following the guidelines of the Universidad
Autónoma del Estado de México (UAEM), as well as Mexican Federal Regulation for Animal
Experimentation and Care (NOM-062-ZOO-2001; governmental approval 143
SGPA/DGVS/02407/13).
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by S. Joy Downes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rivera-Rea, J., González-Morales, J.C., Megía-Palma, R. et al. Seasonal changes in color patches and parasite load of male torquate lizards (Sceloporus torquatus). Behav Ecol Sociobiol 78, 27 (2024). https://doi.org/10.1007/s00265-023-03425-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03425-4