Abstract
Sexual signals serve as an honest indicator of individual quality, reflecting either developmental and/or maintenance costs. A possible underlying physiological mechanism is oxidative stress, which could mediate energy trade-offs between sexual signals and other quality traits. In ectotherms, thermal performance acts as a key indicator of individual quality and influence signal intensity. We investigated how oxidative state is reflected in visual signals of lizards from different thermal habitats. According to our hypothesis, efficient thermoregulation requires different strategies in different thermal environments. In a habitat with predictable temperature changes, animals are less exposed to suboptimal temperature ranges and selection will, therefore, be stronger on the maximum oxidative damage at optimal body temperature. Contrarily, in a habitat with rather stochastic thermal shifts, individuals are often constricted by suboptimal thermal conditions, and oxidative damage can be limiting on a wide temperature range. We used Iberolacerta cyreni and Psammodromus algirus inhabiting stochastic and predictable thermal environments respectively. We examined two aspects of oxidative stress: the level of reactive oxygen metabolites at the preferred temperature (maximal ROM) and the temperature range in which animals produce at least 80% of the maximum level of reactive oxygen metabolites (effective ROM range). In I. cyreni, we found that duller coloration was related to a wider effective ROM range, while expression of coloration in P. algirus was negatively correlated with the maximal ROM. Our results suggest that different thermal constraints affect different aspects of oxidative damage which can indicate individual quality and are, therefore, represented in sexual ornaments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species (ROS) are produced during metabolism and immune processes as by-products of mitochondrial respiration (Halliwell et al. 1985; Korovila et al. 2017) and phagocytic cells’ response against pathogens (Halliwell et al. 1985), and can damage important biomolecules and cause malfunctions (Droge 2002; Halliwell et al. 1985; Korovila et al. 2017). ROS are deactivated by antioxidants (e.g. carotenoids, vitamins C and E) (Burton and Ingold 1984), but their oxidant capacity can sometimes exceed the action of antioxidants. This resulting imbalance in the redox homeostasis is referred to as oxidative stress (OS; Birnie-Gauvin et al. 2017; Korovila et al. 2017; Lemire et al. 2017). Oxidative stress can act as a mediator between physiological state and sexual coloration and can be used to examine evolutionary and ecological questions such as trade-offs between reproductive success and life span or serve as an alternative for the energy-based trade-off conception (Monaghan et al. 2009; Zuk and Stoehr 2002). Previous studies demonstrated that reduced protein intake in rodents decreased mitochondrial respiration and increased lifespan (Fernandes et al. 1976; Sanz et al. 2004). Additionally, oxidative damage negatively affected coloration and consequently reproductive success in fish, reptiles and birds (Cote et al. 2010; Mougeot et al. 2010; Pike et al. 2007). Environmental factors like high temperature also increase levels of metabolic rate and, thereby, level of ROS (Lourdais et al. 2013).
In ectotherms, endogenous regulation of body temperature is limited rendering their physiological performance highly dependent on their environment (Dreisig 1984; Huey and Kingsolver 1993). Performance affects life-history traits such as searching for mates and food, escape from predators, thermoregulation efficiency, reproduction, and survival, and is, therefore, expected to indicate individual quality (Cejudo and Marquez 2001; Ekner-Grzyb et al. 2013; Iraeta et al. 2010; Miles 2004). Many bird and reptile species develop elaborate visual colour signals that affect mate choice (Bennett et al. 1997; Bennett et al. 1996; Rick and Bakker 2008a) and male-male competition (Bajer et al. 2011; Bajer et al. 2010; Perez i de Lanuza et al. 2014; Rick and Bakker 2008b; Whiting et al. 2015). Colour signals can either be created by reflective structures (physical or structural coloration; e.g. ultraviolet [UV], blue) or by pigment molecules (chemical or pigment-based coloration; e.g. orange [carotenoids], black [melanin]); (Kemp et al. 2012). Since these two types of colour signals are linked by strikingly different physiological pathways, they can potentially represent different aspects of individual quality. Structural coloration is more easily developed but poses significant maintenance costs (Perez i de Lanuza et al. 2014) assuring its honesty as a signal. In lizard species, UV and blue patches have been demonstrated to convey information about fighting ability (Bajer et al. 2011), bite force (Martin and Lopez 2009), immune response (Martin and Lopez 2009), parasite load (Molnár et al. 2016) or territory size (Molnár et al. 2016).
Due to developmental and/or maintenance costs, intense nuptial colour badges are only affordable for better quality individuals (Moller and Delope 1994; Weaver et al. 2017). Developmental costs could manifest as lower immune response (Martin and Lopez 2009) or susceptibility to parasites (Salvador et al. 1996), whereas maintenance costs can increase predation risk (Johnson and Candolin 2017) or aggression from conspecifics (Rick and Bakker 2008b). Among lacertid lizards, the European green lizard (Lacerta viridis), the viviparous lizard (Zootoca vivipara), the wall lizard (Podarcis muralis) and the Iberian green lizard (Lacerta schreiberi) bear UV coloration as a sexual signal (Bajer et al. 2012; Martin and Lopez 2009) indicating condition (Martin et al. 2013), parasite infection (Molnár et al. 2013) and territory size (Molnár et al. 2016), influencing mate choice and male-male competition (Bajer et al. 2011; Martin and Lopez 2009; Martin et al. 2015).
On the other hand, intense carotenoid coloration requires high pigment concentration, and since carotenoid precursors can only be acquired from the environment (Grether et al. 2004; Kemp et al. 2012), they are associated with individual quality traits and environmental stress factors (Lopez et al. 2009; Martin and Lopez 2009; Merkling et al. 2016; Pike et al. 2010; Vaclav and Kolnikova 2017). Moreover, carotenoids are also involved in immune reactions as important antioxidants (Halliwell et al. 1985; Korovila et al. 2017) and higher amounts can, therefore, indicate less oxidative damage or a more efficient antioxidant response (Mougeot et al. 2010). Since an immune defence is energetically costly, more resistant individuals with genetically stronger immune system have more energy to invest in their coloration and produce more intensive colour signals (Salvador et al. 1996). Oxidative stress can be a reliable mediator between health status and signalling traits (von Schantz et al. 1999) by connecting environmental factors, optimal performance, immunocompetence and nuptial coloration (Costantini et al. 2009; Peters et al. 2004).
Physiological performance of a lizard is strongly temperature dependent; their performance can therefore be described as the function of temperature (performance curves). These curves are traditionally characterized by Tmax (the temperature of the maximal performance) or Tbreadth (the temperature interval where performance is at least 80% of maximal performance; Bulte and Blouin-Demers 2006; Cejudo and Marquez 2001). It has previously been shown that different populations of the same species can follow different strategies under different thermal conditions (Gabirot et al. 2013). However, to estimate resilience of diverse ectotherm species to changes in climate, we need to reveal coping mechanisms in various thermal habitats.
According to our hypothesis, efficient thermoregulation requires different strategies in different thermal conditions (i.e. in a predictably or in a stochastically changing thermal environment). Such strategies are expected to affect metabolic rate, oxidative stress and expression of nuptial coloration. We propose that lizards in a predictably changing environment should be less exposed to suboptimal temperatures. In this case, selection may be stronger on maximum oxidative damage suffered at optimal body temperature. Better-quality individuals are expected to show lower maximum oxidative damage and brighter signals. In contrast, lizards in a more stochastically changing environment should spend significantly more time at suboptimal temperatures. In this case, selection may be stronger on the temperature range where oxidative damage is still high. We are, therefore, expecting better-quality individuals to display high oxidative damage on a narrower temperature range.
The aim of our study was to examine how oxidative state and nuptial coloration are related in two lizard species from different thermal habitats. Individual quality was estimated by measuring intensity of sexual coloration and health state. To test our predictions, we used two variables depicting different aspects of oxidative damage: the level of reactive oxygen metabolites (ROM) at the optimal temperature, and the temperature interval in which animals suffer at least 80% of the maximal ROM (effective ROM range). We searched for correlation between individual quality and these aspects of oxidative damage.
Material and methods
Study species
The Carpetian rock lizard, Iberolacerta cyreni, is a mountain lacertid lizard native to Spain. The species’ distribution area is the Sierra de Bejar, Sierra de Gredos, La Serrota and Sierra del Guadarrama 1800–2500 m above sea level (Pérez-Mellado et al. 2009). Populations are found above woody areas, in dump, rocky, open scrublands where they occupy areas with inordinate microclimatic conditions—shady, cold cliffs and rocks exposed to direct solar radiation. Previous studies showed that the ventrolateral blue spots expressed by males in the mating season have an important role in intraspecific communication acting as indicators and that more saturated dorsal coloration is correlated with higher reproductive success (Lopez et al. 2004; Salvador et al. 2008). Psammodromus algirus is also a member of Lacertidae, native to France and Spain with a wide distribution from 0 to 2600 m above sea level. Populations are found in various habitats: pine and oak forests or scrublands where temperature fluctuations are buffered by high plant coverage (Mateo et al. 2009). Males develop orange coloration on the head and lateral blue ocelli which are also honest signals of individual quality (Martin and Forsman 1999; Salvador and Veiga 2008).
Field work, morphological measurements, blood sampling
We captured 15 I. cyreni and 15P. algirus males in the mating season of 2016 in central Spain. I. cyreni from ‘Puerto de Navacerrada’ (40° 47′ 04.1″ N, 4° 00′ 44.8″ W; datum = WGS84) and P. algirus from ‘La Golondrina’ (40° 45′ 06.0″ N 4°02′ 03.6″ W; datum = WGS84). All individuals were captured by noosing and transported to the laboratory of the ‘El Ventorrillo’ field station (Museo Nacional de Ciencias Naturales, CSIC) where we conducted all the morphological and spectrophotometric measurements and took blood samples on the day of capture. Lizards were housed individually in plastic tanks (60 × 40 × 40 cm) equipped with a shelter and coconut fibre socket. Optimal temperature (Tday = 28 ± 2 °C, Tnight = 21 ± 2 °C) was maintained by placing all tanks in a heat-controlled chamber; basking spots were provided with spot lamps. Water and food (Tenebrio molitor larvae) were provided ad libitum, and full-spectrum lamps were used for lighting. The mealworms were fed on carrot which contains high concentration of carotenoids (Nicolle et al. 2004). We measured body length (SVL: snout–vent length) with a digital calliper (Mitutoyo, Kawasaki, Japan) and body weight (BW) with an analytical balance (Ohaus Scout Pro SPU-2001, Pine Brook, USA). Ectoparasites (Ixodes spp.) were counted for each individual, and blood samples were collected to estimate blood parasite load (Haemogragarinidae). We collected blood with a sterile 70-μl haematocrit capillary (Hischmann Laborgerate GmbH & CO. KG., Eberstadt, Germany) from the postorbital sinus, and used a drop to create a blood smear. Smears were fixed in methanol for 5 min, then dried and stained with Giemsa solution (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) for 50 min. The number of hemoparasites was calculated for 1000 red blood cells using a microscope to estimate parasite infection intensity.
Colour variables
We measured coloration from 320 to 700 nm with a spectrometer (USB 4000, Ocean Optics, Dunedin, Florida, USA) equipped with RX700 fibre optic and DT Mini 2 GS halogen and deuterium bulb (Ocean Optics, Dunedin, Florida, USA). We used a WS-1 white standard (Ocean Optics, Dunedin, Florida, USA) as a white reference, while the black reference was measured by blocking all incoming light. Colour measurement was performed as detailed in Bajer et al. (Bajer et al. 2010). We recorded three independent measurements from each body part (head, back, throat, belly, flank) of each individual and averaged them separately. We subsequently averaged spectral measurements by 20 nm. We determined the approximate peak regions of each body part by running principal component analysis (PCA) on the spectral curves of each body part with data from all individuals pooled for both species (the peak regions of body parts for both species in the structural/pigment colouration ranges were as follows: throat 320–400 nm/500–650 nm; belly 320–380 nm/550–650 nm; flank 320–490 nm/490–650 nm). To characterize coloration, we calculated the following chromas (the average reflectance on the PCA-derived range divided by the average reflectance of the full spectrum) for each peak of body part: ThroatUV (R320–400/R320–700), ThroatYellow (R500–650/R320–700), BellyUV (R320–380/R320–700), BellyYellow (R550–650/R320–700), FlankUV (R320–490/R320–700), FlankYellow (R490–650/R320–700). In subsequent analyses, we used the above described morphological, parasite load and colour measures as independent variables.
Physiological performance
Before testing the physiological performance tests, the lizards had a 3-day-long acclimatization period. Physiological performance was characterized by performance curves. We first determined the preferred temperature (Tpref) of both species, using a temperature gradient in a rectangular area, where animals were able to move around freely for 60 min. During this period, we measured body temperature on the back of the individual in every 2 min with a laser thermometer (Raytek Raynger ST, Raytek GmbH, Berlin, Germany). Tpref was defined as the average of 30 body temperature measurements. Minimum (Tmin) and maximum temperatures (Tmax) were chosen where individuals no longer gave the righting reflex, following the procedure of Spellerberg (1972) after being cooled down or heated up (with the use of icepacks or lightbulbs, respectively). This part of the experiment was 1 day long. We then defined two further temperatures distributed evenly between Tpref and Tmax and another two between Tpref and Tmin, dividing the temperature scale into six parts. We measured physiological performance for all individuals at all five temperature values and assigned 0 performance value toTmin and Tmax. We set each individual to the desired body temperature (by using icepacks and spot lamps), then placed them into a circular arena, and gently encouraged animals to run until they no longer gave the righting reflex. We registered the distance completed for each individual at each temperature and collected blood samples after every trial using the method described above. Because of the blood sampling after every temperature, the individuals had a 1-day-long resting period. The performance tests on five temperatures were 10 days long. Samples were centrifuged at 7500 rpm for 5 min (Boeco H240, Boeckel & Co, Hamburg, Germany), and plasma was separated to Eppendorf tubes and stored at −20 °C until further analyses.
After all experiments were done, lizards were released at the point of capture. No lizards died or suffered any injury as a result of handling, sampling or treatments.
Measurement of oxidative species
Oxidative status was characterized by the concentration of oxidative metabolites (ROM) in the blood plasma, measured with Diacron d-ROMs Test (Diacron Labs s.r.l., Grosseto, Italy). The tests were conducted following the protocol detailed in Mészáros et al. (2017).
Statistical analysis
We used the statistical method developed for performance curves to establish the oxidative performance along the temperature scale. Thus, we plotted the ROM level measured at each trial against the body temperature of the trial and fitted a curve to the Kumaraswamy function (Cordeiro and de Castro 2011). We determined variables characterizing oxidative performance as maximum ROM (the ROM level measured at the preferred body temperature) and effective ROM range (the ROM interval where the performance is at least 80% of the maximum ROM level = the difference of the two x-values where the y-value is 80% of the maximum y-value).
Normality was tested with Shapiro-Wilk tests and q-q plots. Depending on the results, we used general linear models (GLM) or generalized linear models (GLZ) for normal and non-normal distribution, respectively. For each species, we ran two models with the maximum ROM level or the effective ROM range as dependent variables. Explanatory variables in both models were SVL, condition (residuals from the linear regression of BW against SVL), parasite infection intensity, BellyUV, ThroatUV, FlankUV representing structural coloration and BellyYellow, ThroatYellow, and FlankYellow representing pigment-based coloration. In case of I. cyreni, endoparasite prevalence was 0.86, while that of ectoparasites was 0.0; we therefore used endoparasite infection intensity. InP. algirus, endoparasite prevalence was 0.0, while that of ectoparasites was 0.86; parasite load was therefore characterized by ectoparasite infection intensity. We used GLMs to test connection determinants of maximum ROM level in both species, and effective ROM range in P. algirus, and GLZ to investigate effective ROM range in I. cyreni. We applied backward stepwise model simplification in all models, non-significant explanatory variables were deleted one by one in decreasing order of P, and final models included only the significant main effects. Statistical analyses were performed using R (R Core Team 2016), and oxidative performance curves were created with TableCurve (SYSTAT Software Inc. 2002).
Results
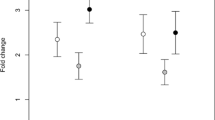
Oxidative metabolite parameters showed significant correlation with signals of individual quality in both species. In I. cyreni, the effective ROM range was negatively correlated with FlankUV (Table 1, Fig. 1), meaning that the individuals suffering substantial oxidative damage on a wider thermal range developed duller ventrolateral UV coloration, whereas maximum ROM level showed no correlation with any examined fitness traits.
In P. algirus, however, the maximum ROM level showed negative correlation with ThroatYellow (Table 2, Fig. 2a); that is, animals suffering a higher level of oxidative damage developed duller throat coloration. BellyUV of P. algirus was also negatively correlated to maximum ROM level (Table 2, Fig. 2b), suggesting that more intense structural coloration appeared on individuals that exhibited lower level of oxidative damage. Furthermore, we also found a positive correlation between maximum ROM level and infection intensity of ectoparasites (Table 2, Fig. 2c), revealing that individuals with a higher parasite load suffered higher oxidative damage.
Discussion
Our results show that different aspects of oxidative damage influence sexual signals in different thermal habitats, in line with the predictions of our hypothesis. In P. algirus, which lives in a more predictable thermal environment, maximum level of oxidative damage was reflected in signals of individual quality, while in I. cyreni, living in a more stochastic thermal habitat, the significant proxy of oxidative status was the effective ROM range. We compared P. algirus preferred temperature (28 °C) to the temperature characteristics of its sampling site (year mean temperature 17.3 °C, maximum mean temperature 22.7 °C, minimal mean temperature 11.8 °C; means during the activity period of lizards—March to October—of the years 1985 to 2010 [ncdc.noaa.gov, 2016a]). It becomes clear that individuals of this species have access to preferred temperatures through long time-periods; the limiting factor, therefore, is not achieving optimal body temperature, but decreasing oxidative damage associated with high physiological performance. Contrarily to the predictable habitat, the thermal parameters of the sampling site of I. cyreni (year mean temperature 10.5 °C, max mean temp 15.1 °C, min mean temp 5.9 °C; means during the activity period of lizards—April to September—of the years 1985 to 2010 (ncdc.noaa.gov, 2016b)) are substantially below the species’ preferred temperature (29 °C), making sufficient physiological performance a great challenge for the individuals. Reaching the adequate body temperature and spending excessive time at suboptimal temperatures can act as stressors, which corresponds with our finding that effective ROM range is the major limiting factor of individual quality in this species.
Higher concentration of oxidative metabolites—potentially higher oxidative damage—was negatively associated with coloration intensity in both species, which suggests that coloration is an honest signal of individual quality. In I. cyreni, individuals bearing duller ventrolateral UV suffered oxidative damage on a wider temperature scale. In lacertid lizards, ventrolateral UV colour has been shown to act as a sexual signal indicating health state as per the interspecific prediction of the Hamilton-Zuk hypothesis (Megía-Palma et al. 2016; Molnár et al. 2013; Rodrigo et al. 2016). Higher levels of reactive species may oxidize pigment molecules which, therefore, result in loss of colouration and signalling (e.g. reduced carotenoid-related immune functions; Garratt and Brooks 2012; Hartley and Kennedy 2004; Metcalfe and Alonso-Alvarez 2010). Oxidative damage can also affect the ultrastructures of UV-green-blue colouration. This may point to the possibility that ventrolateral UV colour is under strong sexual selection and operates as an honest signal of physiological state and tolerance on a wide range of temperatures (Lopez et al. 2004, 2009; Salvador et al. 2008).
Contrary to I. cyreni, we found that the colour variables of P. algirus were correlated not with effective, but with maximum oxidative damage. More intense UV coloration was related to lower level of ROM. High amounts of ROM can have detrimental effects on the ultrastructures creating UV coloration, and thus decrease reflection. According to Carretero (2002), blue spots on the ventrolateral area play an important role in males’ intrasexual communication, so the correlation between colour intensity and oxidative damage suggests that these spots serve as honest signals of individual quality.
Moreover, duller yellow throat coloration was paired with a higher level of ROM in P. algirus. Pigments producing yellow-orange coloration are known for their function as antioxidants (Olsson et al. 2009), which can result in individuals displaying more intense pigment-based coloration having higher amounts of antioxidant pigments and a more effective defence against harmful free radicals (Metcalfe and Alonso-Alvarez 2010). However, further clarification is needed to determine whether pigments are directly involved in neutralizing oxidative species and are, therefore, part of a trade-off between sexual signalling and redox homeostasis, or whether elevated amounts of oxidative species affect coloration indirectly through other undescribed processes.
Finally, we found that P. algirus males with more ticks had a higher level of maximum ROM. This species is known to have a so-called mite pocket (a skin-fold behind the neck rich in vessels) where ectoparasites accumulate in large numbers (Salvador et al. 1999). As part of the inflammatory process induced by tick bites (Goldberg and Bursey 1991), the immune system produces reactive oxidative species in an effort to combat pathogens, but also causing potential damage to the host molecules (Halliwell et al. 1985). Although the number and prevalence of ectoparasites were strikingly high in this species, the same measures for blood parasites were zero. This raises an interesting question since endoparasite (Haemogregarinidae) prevalence in related sympatric species is 13–100% (Molnár et al. 2013), with ticks serving as vectors of the blood parasites (Petit et al. 1990; Smallridge and Paperna 2000). A possible explanation is that elevated levels of ROM due to constant exposure to ectoparasites inhibit blood parasite infection. This would also suggest the possibility of adaptive ‘collection’ of ectoparasites in environments where exposure to endoparasites is high—individuals with continuous immune response combating ectoparasites could be more resistant to endoparasitic infection. Interestingly, Carbayo et al. (2018) found a similar relationship for ectoparasite–endoparasite infection to our finding on these two species, comparing two populations of P. algirus at different altitudes. In their study, they present that the lowland population was highly infected with endoparasites but not with ectoparasites, while for the highland population they found the opposite: low prevalence of endoparasites and high prevalence and infection intensity of ectoparasites. A potential reason could be the lower condition of the lowland population due to habitat differences or climatic conditions.
In conclusion, our results aligned with our hypothesis, since quality signals were correlated with different aspects of oxidative status in thermally different habitats. Average temperature increase or the change of temperature fluctuation can affect the physiological performance of the individuals and the survival of a population or species (Gilbert and Miles 2016; Kubisch et al. 2016). The difference we found, together with the intraspecific variation, can suggest that adaptation to environmental conditions such as temperature can manifest through regulation of oxidative state, which should be considered in further exploring species’ responsiveness and potential adaptive capacity to environmental change.
Based on our current results, we believe that it would be beneficial to look into the molecular composition of these species’ pigment-based coloration and their potential roles as active antioxidants, as well as examining different environmental factors in relation to oxidative state. Involvement of further populations or more species would help to specify selective forces and broaden our understanding of the evolutionary processes acting in populations facing climate change. Nevertheless, the results unequivocally demonstrate that signal intensity and oxidative status are interconnected and are thereby honest proxies of individual quality.
References
Bajer K, Molnár O, Török J, Herczeg G (2010) Female European green lizards (Lacerta viridis) prefer males with high ultraviolet throat reflectance. Behav Ecol and Sociobiol 64:2007–2014
Bajer K, Molnar O, Torok J, Herczeg G (2011) Ultraviolet nuptial colour determines fight success in male European green lizards (Lacerta viridis). Biol Lett 7:866–868
Bajer K, Molnar O, Torok J, Herczeg G (2012) Temperature, but not available energy, affects the expression of a sexually selected ultraviolet (UV) colour trait in male European green lizards. PLoS One 7:e34359
Bennett ATD, Cuthill IC, Partridge JC, Maier EJ (1996) Ultraviolet vision and mate choice in zebra finches. Nature 380:433–435
Bennett ATD, Cuthill IC, Partridge JC, Lunau K (1997) Ultraviolet plumage colors predict mate preferences in starlings. Proc Natl Acad Sci U S A 94:8618–8621
Birnie-Gauvin K, Costantini D, Cooke SJ, Willmore WG (2017) A comparative and evolutionary approach to oxidative stress in fish: a review. Fish Fish 18:928–942
Bulte G, Blouin-Demers G (2006) Cautionary notes on the descriptive analysis of performance curves in reptiles. J Therm Biol 31:287–291
Burton GW, Ingold KU (1984) Beta-carotene - an unusual type of lipid antioxidant. Science 224:569–573
Carbayo J, Martin J, Civantos E (2018) Habitat type influences parasite load in Algerian Psammodromus lizards (Psammodromus algirus). Can J Zool 97:172–180
Carretero MA (2002) Sources of colour pattern variation in Mediterranean Psammodromus algirus. Neth J Zool 52:43–60
Cejudo D, Marquez R (2001) Sprint performance in the lizardsGallotia simonyi and Gallotia stehlini (Lacertidae): implications for species management. Herpetologica 57:87–98
Cordeiro GM, de Castro M (2011) A new family of generalized distributions. J Stat Comput Sim 81:883–898
Costantini D, Dell'Omo G, De Filippis SP, Marquez C, Snell HL, Snell HM, Tapia W, Brambilla G, Gentile G (2009) Temporal and spatial covariation of gender and oxidative stress in the Galapagos land iguanaConolophus subcristatus. Physiol Biochem Zool 82:430–437
Cote J, Meylan S, Clobert J, Voituron Y (2010) Carotenoid-based coloration, oxidative stress and corticosterone in common lizards. J Exp Biol 213:2116–2124
Dreisig H (1984) Control of body-temperature in shuttling ectotherms. J Therm Biol 9:229–233
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Ekner-Grzyb A, Sajkowska Z, Dudek K, Gawalek M, Skorka P, Tryjanowski P (2013) Locomotor performance of sand lizards (Lacerta agilis): effects of predatory pressure and parasite load. Acta Ethol 16:173–179
Fernandes G, Yunis E, Good R (1976) Influence of diet on survival of mice. Proc Natl Acad Sci U S A 73:1279–1283
Gabirot M, Balleri A, Lopez P, Martin J (2013) Differences in thermal biology between two morphologically distinct populations of Iberian wall lizards inhabiting different environments. Ann Zool Fenn 50:225–236
Garratt M, Brooks RC (2012) Oxidative stress and condition-dependent sexual signals: more than just seeing red. Proc R Soc Lond [Biol] 279:3121–3130
Gilbert AL, Miles DB (2016) Food, temperature and endurance: effects of food deprivation on the thermal sensitivity of physiological performance. Funct Ecol 30:1790–1799
Goldberg SR, Bursey CR (1991) Integumental lesions caused by ectoparasites in a wild population of the side-blotched lizard (Uta stansburiana). J Wildl Dis 27:68–73
Grether GF, Kolluru GR, Nersissian K (2004) Individual colour patches as multicomponent signals. Biol Rev 79:583–610
Halliwell B, Gutteridge JMC, Blake D (1985) Metal-ions and oxygen radical reactions in human inflammatory joint disease. Philos Trans R Soc Lond Ser B Biol Sci 311:659–671
Hartley RC, Kennedy MW (2004) Are carotenoids a red herring in sexual display? Trends Ecol Evol 19:353–354
Huey RB, Kingsolver JG (1993) Evolution of resistance to high-temperature in ectotherms. Am Nat 142:S21–S46
Iraeta P, Salvador A, Monasterio C, Diaz JA (2010) Effects of gravidity on the locomotor performance and escape behaviour of two lizard populations: the importance of habitat structure. Behaviour:147133–147150
Johnson S, Candolin U (2017) Predation cost of a sexual signal in the threespine stickleback. Behav Ecol 28:1160–1165
Kemp DJ, Herberstein ME, Grether GF (2012) Unraveling the true complexity of costly color signaling. Behav Ecol 23:233–236
Korovila I, Hugo M, Castro JP, Weber D, Hohn A, Grune T, Jung T (2017) Proteostasis, oxidative stress and aging. Redox Biol 13:550–567
Kubisch EL, Fernandez JB, Ibargungoytia NR (2016) Vulnerability to climate warming of Liolaemus pictus (Squamata, Liolaemidae), a lizard from the cold temperate climate in Patagonia, Argentina. J Comp Physiol B 186:243–253
Lemire J, Alhasawi A, Appanna VP, Tharmalingam S, Appanna VD (2017) Metabolic defence against oxidative stress: the road less travelled so far. J Appl Microbiol 123:798–809
Lopez P, Martin J, Cuadrado M (2004) The role of lateral blue spots in intrasexual relationships between male Iberian rock-lizards, Lacerta monticola. Ethology 110:543–561
Lopez P, Gabirot M, Martin J (2009) Immune challenge affects sexual coloration of male Iberian wall lizards. J Exp Zool A Ecol Genet Physiol 311A:96–104
Lourdais O, Guillon M, Denardo D, Blouin-Demers G (2013) Cold climate specialization: adaptive covariation between metabolic rate and thermoregulation in pregnant vipers. Physiol Behav 119:149–155
Martin J, Forsman A (1999) Social costs and development of nuptial coloration in male Psammodromus algirus lizards: an experiment. Behav Ecol 10:396–400
Martin J, Lopez P (2009) Multiple color signals may reveal multiple messages in male Schreiber's green lizards, Lacerta schreiberi. Behav Ecol Sociobiol 63:1743–1755
Martin M, Meylan S, Gomez D, Le Galliard JF (2013) Ultraviolet and carotenoid-based coloration in the viviparous lizard Zootoca vivipara (Squamata: Lacertidae) in relation to age, sex, and morphology. Biol J Linn Soc 110:128–141
Martin M, Meylan S, Perret S, Le Galliard JF (2015) UV coloration influences spatial dominance but not agonistic behaviors in male wall lizards. Behav Ecol Sociobiol 69:1483–1491
Mateo JA, Cheylan M, Nouira MS, Joger U, Sá-Sousa P, Pérez Mellado V, Martinez Solano I, Sindaco R (2009) Psammodromus algirus (errata version published in 2016). The IUCN Red List of Threatened Species 2009: e.T61558A86629654. https://doi.org/10.2305/IUCN.UK.2009.RLTS.T61558A12491246.en.
Megía-Palma R, Martínez J, Merino S (2016) A structural colour ornament correlates positively with parasite load and body condition in an insular lizard species. Sci Nat 103:52. https://doi.org/10.1007/s00114-016-1378-8
Merkling T, Hamilton DG, Cser B, Svedin N, Pryke SR (2016) Proximate mechanisms of colour variation in the frillneck lizard: geographical differences in pigment contents of an ornament. Biol J Linn Soc 117:503–515
Mészáros B, Herczeg G, Bajer K, Török J, Molnár O (2017) Effects of energy and thermoregulation time on physiological state and sexual signal in a lizard. J Exp Zool A 327:570–578
Metcalfe NB, Alonso-Alvarez C (2010) Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct Ecol 24:984–996
Miles DB (2004) The race goes to the swift: fitness consequences of variation in sprint performance in juvenile lizards. Evol Ecol Res 6:63–75
Moller AP, Delope F (1994) Differential costs of a secondary sexual character - an experimental test of the handicap principle. Evolution 48:1676–1683
Molnár O, Bajer K, Mészáros B, Török J, Herczeg G (2013) Negative correlation between nuptial throat colour and blood parasite load in male European green lizards supports the Hamilton-Zuk hypothesis. Naturwissenschaften 100:551–558
Molnár O, Bajer K, Szövényi G, Török J, Herczeg G (2016) Space use strategies and nuptial color in European green lizards. Herpetologica 72:40–46
Monaghan P, Metcalfe NB, Torres R (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12:75–92
Mougeot F, Martinez-Padilla J, Blount JD, Perez-Rodriguez L, Webster LM, Piertney SB (2010) Oxidative stress and the effect of parasites on a carotenoid-based ornament. J Exp Biol 213:400–407 ncdc.noaa.gov, 2016a. Granada weather. ncdc.noaa.gov, 2016b. Navacerrada weather
Nicolle C, Simon G, Rock E, Amouroux P, Rémésy C (2004) Genetic variability influences carotenoid, vitamin, phenolic, and mineral content in white, yellow, purple, Orange, and dark-orange carrot cultivars. J Am Soc Hortic Sci 129:523–529
Olsson M, Wilson M, Isaksson C, Uller T (2009) Polymorphic ROS scavenging revealed by CCCP in a lizard. Naturwissenschaften 96:845–849
Perez i de Lanuza G, Carazo P, Font E (2014) Colours of quality: structural (but not pigment) coloration informs about male quality in a polychromatic lizard. Anim Behav 90:73–81
Pérez-Mellado V, Cheylan M, Martínez-Solano I (2009) Iberolacerta cyreni. The IUCN red list of threatened species 2009: e.T61514A12498292. https://doi.org/10.2305/IUCN.UK.2009.RLTS.T61514A12498292.en. Accessed 26 Aug 2019
Peters A, Denk AG, Delhey K, Kempenaers B (2004) Carotenoid-based bill colour as an indicator of immunocompetence and sperm performance in male mallards. J Evol Biol 17:1111–1120
Petit G, Landau I, Baccam D, Lainson R (1990) Description et cycle biologique d'Hemolivia stellata ng, n. sp., hémogrégarine de crapauds brésiliens. Ann Parasitol Hum Comp 65:3–15
Pike TW, Blount JD, Bjerkeng B, Lindstrom J, Metcalfe NB (2007) Carotenoids, oxidative stress and female mating preference for longer lived males. Proc R Soc B 274:1591–1596
Pike TW, Blount JD, Lindstrom J, Metcalfe NB (2010) Dietary carotenoid availability, sexual signalling and functional fertility in sticklebacks. Biol Lett 6:191–193
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Rick IP, Bakker TCM (2008a) Color signaling in conspicuous red sticklebacks: do ultraviolet signals surpass others? BMC Evol Biol 8:189. https://doi.org/10.1186/1471-2148-8-189
Rick IP, Bakker TCM (2008b) Males do not see only red: UV wavelengths and male territorial aggression in the three-spined stickleback (Gasterosteus aculeatus). Naturwissenschaften 95:631–638
Rodrigo MP, Javier M, Santiago M (2016) Structural- and carotenoid-based throat colour patches in males of Lacerta schreiberi reflect different parasitic diseases. Behav Ecol Sociobiol 70:2017–2025
Salvador A, Veiga JP (2008) A permanent signal related to male pairing success and survival in the lizard Psammodromus algirus. Amphibia-Reptilia 29:117–120
Salvador A, Veiga JP, Martin J, Lopez P, Abelenda M, Puerta M (1996) The cost of producing a sexual signal: testosterone increases the susceptibility of male lizards to ectoparasitic infestation. Behav Ecol 7:145–150
Salvador A, Veiga JP, Civantos E (1999) Do skin pockets of lizards reduce the deleterious effects of ectoparasites? An experimental study withPsammodromus algirus. Herpetologica 55:1–7
Salvador A, Diaz JA, Veiga JP, Bloor P, Brown RP (2008) Correlates of reproductive success in male lizards of the alpine species Iberolacerta cyreni. Behav Ecol 19:169–176
Sanz A, Caro P, Barja G (2004) Protein restriction without strong caloric restriction decreases mitochondrial oxygen radical production and oxidative DNA damage in rat liver. J Bioenerg Biomembr 36:545–552
Smallridge C, Paperna I (2000) Ultrastructure of Hemolivia mariae gamonts in the blood of the lizardTiliqua rugosa and their development to oocyst stage in the tick Amblyomma limbatum. Parasitol Res 86:563–569
Spellerberg IF (1972) Temperature tolerances of southeast Australian reptiles examined in relation to reptile thermoregulatory behaviour and distribution. Oecologia 9:23–46
SYSTAT Software Inc (2002) TableCurve 2D Version 5.01
Vaclav R, Kolnikova Z (2017) Effects of food and thermal regimes on body condition indices and skin colouration in corn snakes. Biologia 72_84–95
von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H (1999) Good genes, oxidative stress and condition-dependent sexual signals. Proc R Soc B 266:1–12
Weaver RJ, Koch RE, Hill GE (2017) What maintains signal honesty in animal colour displays used in mate choice? Philos trans Royal Soc B 372. https://doi.org/10.1098/rstb.2016.0343
Whiting MJ, Noble DWA, Somaweera R (2015) Sexual dimorphism in conspicuousness and ornamentation in the enigmatic leaf-nosed lizard Ceratophora tennentii from Sri Lanka. Biol J Linn Soc 116:614–625
Zuk M, Stoehr AM (2002) Immune defense and host life history. Am Nat 160:S9–S22
Acknowledgements
The authors are thankful for the help of the Behavioural Ecology Group at Eötvös Loránd University and the staff and facilities at El Ventorrillo Field Station (MNCN-CSIC).
Funding
Open access funding provided by Eötvös Loránd University (ELTE). This work was supported by the Ministerio de Economía e Innovación (Spain; MINECO CGL2014-53523-P); the National Research, Development and Innovation Office (Hungary; K 115 970).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the Environmental Agency of the Community of Madrid local government (licence number: 10/056780.9/16).
Additional information
Communicated by: Matthias Waltert
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mészáros, B., Jordán, L., Bajer, K. et al. Relationship between oxidative stress and sexual coloration of lizards depends on thermal habitat. Sci Nat 106, 55 (2019). https://doi.org/10.1007/s00114-019-1649-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-019-1649-2