Abstract
Sexual conflict has long been perceived as a solid background for the evolution of parental care. Recent studies, however, highlight the importance of cooperation between the parents, especially in socially monogamous systems. Here, we examined parental performance of a small monogamous passerine with bi-parental care, the Eurasian Reed Warbler (Acrocephalus scirpaceus, RW), looking at the issue from the perspective of parents’ cooperation. Using accurate and non-invasive video data on parent chick provisioning from 78 nests varying in brood size, we found some evidence of parents cooperation: both parents similarly adjusted their feeding rate to brood size, and the higher was their both contribution, the better was chicks condition (expressed by defecation rate). However, contrary to our expectations (based on premises from similar studies on other bird species) we did not find evidence for frequent and active synchronisation of nest visits. Importantly, we found a negative relationship between synchronisation level and chick body condition, suggesting that synchronisation may actually not be that beneficial in the study system. The results are surprising and highlight the importance of studying various species to understand mechanisms behind cooperation of partners in the bi-parental care system.
Significance statement
Biparental care has long been viewed in the context of conflict between the breeding partners, and only recently they are considered cooperating agents caring together for the common offspring. Nevertheless, studies applying such a perspective are still scarce and there is a pressing need to test different species. Using video data on chick provisioning by Reed Warbler parents, we found evidence of partner cooperation: both parents adjusted their feeding rate to the number of nestlings, and the higher was their joint contribution, the better was offspring condition. Parents did not synchronise their nest visits more compared to randomly generated visits, but we found that synchronisation level negatively affected chick condition. Our results highlight the need to consider various species to fully understand mechanisms behind cooperation of breeding partners.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since Triver’s (1972) seminal paper, parental investment has been viewed through the perspective of sexual conflict between parents. The conflict is assumed to arise because males and females have different reproductive interests, mostly due to the differences in the quality and quantity of their gametes (Clutton-Brock and Vincent 1991). It has been proposed that the evolutionary force related to these sexual differences is also the strongest in shaping the overall parenting strategy (Trivers 1972). In this context, the sex limited in the number of produced offspring (thus, females producing a low number of large gametes or males in the condition of male-biased sex ratio in the population) would primarily benefit from investing in the current offspring, for example, in the form of post-zygotic parental care. In contrast, the sex less constraint in the number of produced offspring (thus, males producing large numbers of low-cost gametes or females in the condition of female-biased sex ratio in the population) would increase their fitness by mating with multiple partners, not caring much for the offspring (Trivers 1972). Although in some animals this line of reasoning holds true, in many species gamete production constitutes only a tiny component of parental investment (Kokko and Jennions 2008), and initial sex differences may not be the only selective force shaping general reproductive strategy.
Reproductive systems with bi-parental care particularly challenge the question of sexual conflict over parental care. In this system, male and female work together to achieve a common goal, i.e. successfully raised offspring, and they apparently cooperate. Here, the initial sexual conflict is supposed to be enhanced because benefits (breeding success), resulting from the combined effort of two parents, are shared by the parents, while costs (reduced survival and future reproduction of the parent) are paid individually, being related to individual parental effort (Trivers 1972; Chase 1980). Thus, each parent is expected to monitor and behaviourally respond to the partner’s behaviour (negotiating amount of parental effort), as only in this way bi-parental care strategy can be evolutionary stable (Houston et al. 2005; Johnstone and Hinde 2006; Johnstone 2011; Johnstone and Savage 2019). For instance, a male would provide parental care only when being “certain” of female commitment, otherwise a female might free ride on male’s effort, which in turn could lead to erosion of the whole parenting strategy to uniparental care.
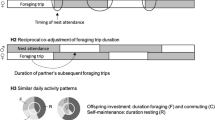
Despite sexual conflict being inevitable, due to different evolutionary interests of the two sexes, natural selection can promote the evolution of parental cooperation (Griffith 2019; Servedio et al. 2019; Wojczulanis-Jakubas 2021). One of the drivers of parental cooperation may be associated with fair contributions from the two parents that keep them both in an optimal zone of their parental effort. That, in turn, would increase not only a chance of their survival and future breeding of both partners (particularly important for long-lived species and/or species of high-mate fidelity) but also a chance of success in the current breeding attempt (Wojczulanis-Jakubas 2021). Recent studies clearly show that parental birds not only cooperate in the sense of taking care for the common offspring, but also adjust their parental activities in respect to each other in a way that may maximise their breeding outcome (as reviewed in Griffith 2019). If the predation pressure is the main constraint, parents may synchronise their nest visits to reduce overall activity around the nest, limiting nest exposure to potential predators. Indeed, synchronisation of feeding visits of Blackcap (Sylvia atricapilla) parents significantly decreased parental activity at the nest and increased nest survival rate (Leniowski and Węgrzyn 2018a). Parents may also coordinate their visits to optimise food delivery, which should promote development of the offspring (Griffith 2019; Servedio et al. 2019; Wojczulanis-Jakubas 2021). For example, zebra finch (Taeniopygia guttata) fledglings had higher body mass if their parents adjusted nest visits in a way to perform the feeding in a synchronised manner (Mariette and Griffith 2015; Leniowski and Węgrzyn 2018a, b; Baldan and Ouyang 2020). Although the idea to examine parental care in the perspective of parental coordination is quite recent (reviewed by Griffith 2019), studies published so far have already provided considerable support. At the same time, the existing studies demonstrate a great variety of patterns and level of parental coordination, as well as its effect on fitness. Thus, to fully understand how common are different patterns of parental coordination and what are their correlates, it is necessary to screen various species in various contexts.
In this study we examined chick provisioning by the Eurasian Reed warbler Acrocephalus scirpaceus (thereafter RW), looking at the parental behaviour from the perspective of parents’ cooperation and coordination. The RW is a small bird species, with bi-parental care system. Both male and female provision chicks and they do it at similar rate (Brown and Davies 1949; Duckworth 1990; LH unpublished data), which predisposes the species for examining parental cooperation. The brood size of the RW may range from 1 to 5 chicks (Halupka et al. 2021), which in turn creates a natural, experiment-like set up for examining parental response to different levels of brood needs and/or provisioning patterns. Nest predation is an important factor affecting reproduction success of the species in the study population (Halupka et al. 2014). It can potentially be another driver of coordination of parental activities (Leniowski and Węgrzyn 2018a); however, we have not tested its effect in the present study due to insufficient data (see details in Methods section).
We asked five questions and hence formulated five predictions. First, we asked whether partners cooperate by increasing their feeding effort if necessary. For this purpose, we leveraged the advantage of data set consisting of nests of different brood sizes, which could represent different brood needs. Larger broods having proportionally larger needs were expected to be provisioned more than small broods. Assuming parental cooperation, we expected both sexes to respond to brood needs in a similar way, by exhibiting a similar feeding rate in respect to the brood size. Second, we analysed a relationship between feeding rate of male and female parents in respect to each other (when controlled for brood size). We expected such a relationship to be significant and positive if the two parents respond to brood needs and environmental circumstances in a similar way (Baldan and van Loon 2022). No correlation between male and female feeding rate would indicate a lack of mutual male and female interactions in parental performance. Third, to examine the adaptive value of parental cooperation, we considered the effect of the overall effort of parenting pair on chicks’ condition. If parental cooperation could be a selective force, we expected a positive relationship between parental effort and the condition of the brood. We used the rate of faeces production by chicks in the nest (hereafter defecation rate) as a proxy for chicks current condition, as it is known to correlate with digestive efficiency (Kilner 2001) and nutritional state (Karasov and Wright 2002). Fourth, we examined how much RW parents coordinate their activity by temporally synchronising their feeding visits, and how this pattern differed from what could be expected by chance. Evidence of behavioural adjustments (such as synchronisation of the nest visits) could indicated on active parents cooperation (Griffith 2019). Finally, we considered the adaptive value of the nest visit synchronisation. Temporal synchronisation of nest visits may be beneficial and so favoured by natural selection. This is because parents visiting the nest at the same time may mutually control the partner performance (which could minimise the sexual conflict over the parental care (Mariette and Griffith 2015; Baldan and Griggio 2019)). Concomitantly, parents visiting the nests at the same time may better distribute the food among the nestlings (Shen et al. 2010). By synchronising nest visits, parents may also decrease the disturbance around the nest site, which may reduce the risk of predation (Martin and Briskie 2009; Ghalambor et al. 2013; Hua et al. 2014). Although existing adaptive explanations of nest visit synchronisation are not mutually exclusive and overall hard to disentangle without experimental manipulations, we analysed synchronisation in respect to overall feeding rate and defecation rate, expecting to find a significant positive relationship between the two. Then, we expected that if synchronisation is somehow related to overall efficiency of feeding in the nest, there would be a positive relationship between synchronisation and defecation rate.
Methods
None of the methods applied in the study was blind as the study involved individually marked birds, and knowing their identity was important for recording the behaviour.
Fieldwork
Data were collected during the fieldwork performed in the Stawy Milickie nature reserve (51.5387 N, 17.3401 E), SW Poland. The local population, inhabiting area of ca 3 ha, has been studied since 1970, with birds colour marked since 1980. This enabled us to locate sufficient number of nests and follow parental behaviour at the nest (otherwise hard to perform in such an inconspicuous species as the RW). In each study season ca. 85% of RW nests are found at the building stage and followed after. The nests were visited every 2 days or daily (before the expected hatching day, to accurately determine the age of chicks). If a nest was found after hatching, chicks were aged based on morphometrics and/or overall chick appearance, following (Hałupka et al. 2018). Other details about field procedures can be found elsewhere (Orłowski et al. 2016a, b; Halupka et al. 2021). For the purpose of the present study, the parental behaviour was video recorded in three consecutive breeding seasons (2020–2022). The recording was performed on the eighth day of the chick’s life. Almost all nests that survived until this stage were video recorded, but the good quality data were obtained for a total of 78 nests (37, 21, and 20 in 2020, 2021, and 2022, respectively). Commercial cameras (JVC Everio GZ330) were set up on tripods, ca 1 m in front of the nest (usually in a path closest to the nest) and zoomed and centred on nest and the most proximate surroundings (radius of ca 0.5 m). Both parents were individually marked with a combination of metal and colour-plastic rings. After capturing, individuals were also sexed, based on the examination of cloaca protuberance and/or brood patch (Svensson 1992). The eighth day of the chick rearing was chosen for the video recording as at this age chicks are becoming thermally independent, and so females rarely brood them during the day (LH unpublished data), being able to fully engage in chick provisioning. Same age of the chicks in all the nests reduced a potential effect of chick age on the examined variables. All the videos were recorded for on average of 2.3 h (range: 1.1–5.4 h) starting before noon, and performed in a similar, good weather conditions (no or slight wind, no rain). Brood size in the considered data set ranged from 1 to 5, with median of 4 chicks, with no significant inter-annual differences in frequency of number of chicks (X-squared = 7.86, df = 8, p = 0.45; Supplementary Materials: Fig. S1).
Video analysis
We processed the video material in BORIS software (Friard and Gamba 2016), marking all the nest visits as a state event (i.e. time interval, with starting and ending timestamps), and feedings and faeces collection as a point event (i.e. a single timestamp). For each parent all the events were marked separately. As reported in literature for some other passerines, e.g. Quan et al. (2015), defecation of RW chicks coincides with a feeding event (this is always a single defecation of a single chick; it does not occur at every feeding event and defecating chick is not necessarily the one being just fed) and faeces were collected by the parent; thus, the rate of the faeces collection reflected defecation rate in the nest. Defecating chicks put their rump out of the nest; thus, all defecation events could be detected. The time accuracy of the marking allowed in BORIS is set to three decimal points of a second but our analysis of inter- and intra-observer difference (not presented here) indicated that this precision should be rounded to 1 s.
Statistical analysis
Due to varying duration of recordings that could potentially affect the feeding and defecation rate (if the number of feedings/defection events changes over the time, for example, intensive feeding/defecation rate exhibited in first hour may be followed by decreased rate of both in the second hour of the recording), to calculate the rate for each variable we applied two approaches: 1) we divided the total number of feedings/defecation events per duration of a recording (relaxed approach that allows to use maximum of the collected data), and 2) we calculated the number of feedings/defecation events for each complete hour of recording separately (more conservative approach, that could help to “standardise” all the recordings, if needed). Using data derived in the second approach and for all the nests that were recorded for at least 2 h, we calculated repeatability of feeding and defecation rates in the nest, to examine the effect of hour of the recording. To do so, we run two separate Poisson models (with log link function) with a number of feedings or faeces removal performed by an individual (regardless of the sex) as a response variable, and the bird/nest identity (multiple records for an individual/nest) as a random effect. In the model with the number of feedings we also included bird sex as a fixed effect. The number of iterations both for bootstrap and permutation tests was set at 500. The models were built following recommendation of Nakagawa and Schielzeth (2010) and repeatability of behaviours was calculated using rptR package (Stoffel et al. 2017). Since non-zero repeatability was revealed both for feedings [original-scale approximation: R = 0.284, SE = 0.066, CI = [0.145, 0.403], P (LRT) < 0.001, P (permutation) = 0.002] and defecation rate [original-scale approximation: R = 0.369, SE = 0.092, CI = [0.139, 0.492], P (LRT) = 0.001, P (permutation) = 0.006], in further analyses of feeding and defecation rate, we applied the rate calculated using the relaxed approach.
Since we found no significant inter-seasonal differences for parental effort being expressed as the feeding rate when controlled for the brood size (Supplementary materials: Fig. S2), for simplicity of the analysis data from all three seasons were pooled, and the season was not considered in any of subsequent analyses.
To examine similarity between males’ and females’ response to brood needs, we considered feeding rate exhibited by male and female parents controlled for the brood size. To account for effect of brood size we divided feeding rate (calculated using the relaxed approach, as described above) by the number of chicks in the nest. Thus, we examined parental performance per nestling (i.e. not an absolute effort in response to the brood size). Using this response variable we ran a linear model with brood size (number of chicks treated as factor), sex of the parent, and interaction between the two as explanatory variables. We log-transformed the response variable to normalise residual distribution.
To test the prediction about a positive relationship between the feeding rate of male and female parent, we performed a correlation analysis using the brood-size-standardised feeding rates of the two parents in particular nests. To test the significance of the observed correlation coefficient we performed Monte Carlo randomisation. To this end, we sampled with replacement the standardised feeding rates of each sex separately and calculated the correlation coefficient for the randomised pairs of male and female data. Iterating procedure 1000 times, we received a normal distribution of correlation coefficients generated by chance. Subsequently, we compared the randomised coefficients with the observed value, calculating p-value as the proportion of randomised coefficients that would be equal to or lower than the observed one.
To examine the effect of overall parental effort on the chicks’ current condition, we considered feeding rate exhibited by both parents (the number of male and female feedings pooled; proxy for parental effort in the nest) and defecation rate in the nest (proxy for chicks’ current condition), both being standardised by the brood size (i.e. divided by the number of chicks in the nest). Then, we ran correlation analysis between the two variables and tested significance of the coefficient with the randomisation procedure (as for the testing correlation between male and female feeding rate, see above).
To test whether parents tend to temporally synchronise their feeding visits, we first calculated observed proportion of nest visits when partners met. Then, we applied Monte Carlo randomisation to test how the observed proportion is different from the one that could be expected by chance. Before any calculation, to time interval of each nest visit we added a time margin of 30 s at each side of the interval, to account for a possible partners’ encounter outside the camera view. This two-side margin makes in total of 1 min, and follows recommendations of Leniowski and Węgrzyn (2018a). As in the system of blackcaps (Leniowski and Węgrzyn 2018a), we had numerous observations that RW parents often arrive together with food, but then one of them perches in close vicinity of the nest waiting for the other to complete feeding. Thus, the added time buffer accounts for subsequent visits of the partners, performed shortly one after the other. In other words, the visit was considered to be synchronised if two partners met at the nest or paid their visit within 30 s time interval in respect to each other. For randomisation, we first shuffled the observed sequences of nest visits and inter-visit intervals (with the added time margins of 30 s to the nest visits), then we calculated the proportion of feeding visits when the two parents encountered in the randomised patterns. The procedure was repeated 1000 times, resulting in 1000 values of the randomised proportions for each pair. Using this randomised distribution, we calculated a p-value for every nest observation. To obtain an overall p-value for the whole data set, we used the Fisher method, using the R package metap (Dewey 2023).
Using observed and randomised data sets, we calculated the synchronisation index for each pair as the proportional difference between the observed (obs) and expected (exp) proportion of nest visits with partners’ encounter (the formula: [obs-mean(exp)] × mean(exp)−1). The interpretation of the index is that its positive values indicate partners’ synchronisation, with higher values denoting stronger synchronisation. Zero values indicate random performance of partners, while negative values indicate that avoid synchronisation and rarely meet at the nest area. We considered the value of the index as the intensity of the synchronisation.
Finally, to consider possibility of parents’ synchronisation of the nest visits to be adaptive, we examined how the synchronisation index is related to the brood size. For this purpose, we run a simple linear model with synchronisation index as a response variable and the number of chicks treated as a factorial explanatory variable. We also examined relationship between the synchronisation index and the feeding rate performed at the nest and the chick condition (expressed by brood-size-standardised defecation rate in the nest). To do so, we ran two separate linear models with the feeding or defecation rate as the response variables (both log-transformed to normalise residuals) and the index of synchronisation as a fixed effect in both models. Although overall predation rate was quite high in the study population (44% of 254 monitored nests were predated over the study period, LH unpublished data), potential effect of predation pressure on parent’s nest visits synchronisation could not be tested here, as of all the nests considered in the present study only one was predated after video recording.
Results
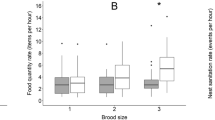
The model examining brood-size-standardised feeding rate exhibited by male and female explained 16% of variance and was significant (F = 4.172, df = 9 and 146, p < 0.001). There was no difference between the sexes in brood-size-standardised feeding rate in respect to the brood size (F = 2.43, df = 1, P = 0.24). Sex interaction with the brood size was also insignificant, F = 0.83, df = 4, P = 0.42) but brood size was significant on its own (F = 8.06, df = 4, P < 0.001). The feeding rate was the lowest at the largest broods, and that was particularly well visible in males (Fig. 1), but since sex was not significant while being the core of the question, we did not apply any post-hoc testing.
Brood-size-standardised feeding rate (number of feedings performed per hour per chick) of female (orange) and male (olive) parents at particular brood size classes (1 to 5 chicks in the nest). Boxes indicate the inter quartile range (IQR), with the central line depicting the median and the whiskers extending to 1.5 × IQR. At the right of the boxes frequency distributions are shown. Points indicate raw data. No statistically significant interaction between the sex and brood size
Brood-size-standardised feeding rate of male and female parents was significantly correlated (r = 0.31, P (permutation) = 0.003; Fig. 2).
Correlation between brood-size-standardised feeding rate (number of feedings per hour per chick) of male and female parents (left panel) and significance of the correlation coefficient tested in a randomisation procedure (right panel)—the observed value of correlation coefficient (red vertical line), being clearly beyond the distribution of randomised values (grey filled), indicates strong and not random correlation between feeding rate of male and female parents
Brood-size-standardised feeding rate of parenting pair was correlated with brood-size-standardised defecation rate in the nest (r = 0.54, P (permutation) < 0.001; Fig. 3).
Correlation between brood-size-standardised defecation rate (number of defecations per hour per chick) and brood-size-standardised feeding rate in the nest (number of feedings per hour per chick) (left panel), and significance of the correlation coefficient tested in a randomisation procedure (right panel)—the observed value of correlation coefficient (red vertical line) being clearly beyond the distribution of randomised values (grey filled) indicates strong and not random correlation between feeding rate and defecation parent
Parents met at the nest on average at 25% (range: 4–46%) of all the feeding visits observed in the nest. Randomisation procedure revealed that the observed patterns of parents’ encounters at the nest were not much different from that what could be expected by chance (Fisher test, X-squared = 152.11, df = 154, p = 0.53), with synchronisation index ranging from − 0.58 to 1.20 and median being − 0.001 (Fig. 4). The synchronisation index was not related to the brood size (F = 1,37, df = 4, p = 0.25).
Distribution of the synchronisation index observed in the considered data set. The index calculated as the proportional difference between the observed and expected proportion of nest visits with partners’ encounter, with the expected value established in a randomisation procedure. The interpretation of the index is that its positive values indicate partners’ synchronisation, with higher values denoting stronger synchronisation of the nest visits of the two parents
The model with brood-size-standardised feeding rate considered in relation to synchronisation index explained only 1% variance and was insignificant (F = 2.643, df = 1, 76, p = 0.11) but there was a trend of negative relationship between the two (Fig. 5, Table 1). The model with brood-size-standardised defecation rate considered in relation to synchronisation index explained 12% of variance and was significant (F = 11.67, df = 1, 76, p = 0.001). Consequently, the negative estimate for the relationship between brood-size-standardised defecation rate and synchronisation index was significant (Fig. 5, Table 1).
Relationship between synchronisation index and brood-size-standardised feeding rate of the pair (number of feedings per hour per chick; left panel) and brood-size-standardised defecation rate in the nest (number of defecations per hour per chick; right panel); values of the response variables not being log-transformed on the plot (as they were in the model). Significant results denoted with solid black regression line and insignificant with dashed grey line. Grey areas denote 95% confidence interval for the regression line
Discussion
Examining parental performance of the Reed warbler males and females during the chick provisioning phase, we found that both sexes responded similarly to brood needs. They both adjusted (i.e. increased) the number of feedings with increasing brood size, so chicks received a similar amount of food from parents, regardless of number of nestlings in the nest. The feeding effort of the parents was apparently not sufficient at the brood size of five chicks, as then, brood-size-standardised feeding rate was lower but there was no difference between the sexes in this response. In general, the feeding rate (when controlled for the brood size) of parenting partners was positively correlated, and high feeding rate in the nest was associated with better current condition of the brood, as indicated by the positive correlation between feeding and defecation rate in the nest. Interestingly, and contrary to our expectations, the synchronisation of feeding visits of the two parents did not occur more frequently than it could be expected by chance. There was some inter-pair variation in the level of synchronisation of parents’ nest visits, and we found that higher level of the synchronisation is related to lower defecation rate in the nest.
We expected that in a species with biparental care such as the RW, cooperation between the parents could be observed in a positive correlation between male and female parental effort. Our results indicate that indeed it may be the case. However, the mechanism behind this relationship cannot be determined based on the present study due to its correlational character. There may be two interpretations of the observed correlation in terms of behavioural interactions between the parents. First, it may be an effect of conditional cooperation, where feeding rate of one parent is a reciprocal response to the partner’s performance. As such it could be a manifest of a sexual conflict between the parents over the parental care (Johnstone et al. 2014). Possibility of multiple breeding attempts (up to five, Schulze-Hagen et al. 1996), including partners exchange over the breeding season (Borowiec 1992), could weaken the value of current breeding attempt, and that might further enhance the sexual conflict. Second possibility is that the positive correlation between male and female parental effort is an effect of partners’ similarity in foraging efficiency (Baldan and van Loon 2022); thus, both may simply feed their chicks as frequently as it is only possible in given circumstances. Then, low values of the feeding rate in some pairs might be simply an effect of some variables that we could not control in the study (e.g. temporally lower food availability limiting foraging efficiency of both sexes; similarly low body condition of the two parents due to assortative mating). Clearly, further studies (including an experimental approach) are needed to examine causality of the observed correlation, and the inter-pair variability in the similarity of the partners in the feeding rate may provide a powerful background for the investigation.
Our results show that chick feeding rate exhibited by the parenting pair is positively correlated with defecation rate in the nest. We assumed that defecation rate in the nest could be a proxy of current brood condition. We based our assumption on the fact that defecation rate in birds is known to correlate with digestive efficiency (Kilner 2001) and nutritional state (Karasov and Wright 2002). Then, defecation rate in the nest was positively related to feeding rate of the parents in our study system. Importantly, defecation rate was measured at the same time as the parental behaviour; thus, the link between the two is more straightforward, i.e. not much affected by the time elapsed between the two measurements (as it could be the case for fledging success, for example). Possibility that higher defecation rate is an effect of digestion problem is unlikely to bias the results given a big sample size, we considered in the present study. In the context of the material collected for the present, non-invasive study, defecation rate was the only available variable for the offspring development. Regular measurements of chicks’ body mass could be an alternative here but could also increase the risk of nest predation, and that not only could affect the parents’ behaviour but also jeopardise the breeding success. Also, considering fledging success as a measurement of the effect of the total parental effort was not an option, as when accounting for external mortality (predation), there was little variation in this variable in the monitored nests (chicks fledged successfully in 94% nests). Although defecation rate may not be a conventional measurement, we believe it gives some insight into the adaptive value of the parental performance. The link between current chicks’ condition and their overall fitness is to be verified but our results suggest already that similarly high effort of both parents may promote chicks’ development. This, in turn, suggests that parents’ cooperation may be beneficial, and so could be a selective force shaping bi-parental care strategy (Griffith 2019; Servedio et al. 2019).
Cooperating parents may be expected to mutually adjust their behaviours, to further enhance the offspring fitness. Coordination of parental activities has been recently suggested as a manifestation of tight parents’ cooperation, and in some species (of parenting strategy similar to RW) parents have been reported to actively synchronise their nest visits (57–67% of parents nest visits synchronised in the blackcaps (Leniowski and Węgrzyn 2018a), 63% in the crimson Rosella Platycercus elegans (Krebs et al. 1999), 73% in the long-tailed finch Poephila acuticauda (Rooij and Griffith 2013), and 78% in the zebra finch (Mariette and Griffith 2012). This synchronisation was also reported to be beneficial for the offspring (e.g. increasing their survival, Mariette and Griffith 2012). Thus, we expected RWs to synchronise their visits. Contrary to this expectation, we found that only one-fourth of the nest visits were synchronised, and this value was not different from that what could be expected by chance, given exhibited number of feedings and duration of nest and inter-nests visits time intervals. This result contrasts with expectations but it is not entirely surprising as no evidence of parents’ coordination, despite evident circumstances favouring such a pattern, has already been reported (Enns and Williams 2022). All these contrasting results highlight how important it is to consider various species before generalising patterns and to understand mechanisms behind synchronisation.
Although the level of nest visit synchronisation in the RW was generally low, there was considerable inter-pair variation, and there were partners that synchronised their visits quite much and those which tended to avoid such a synchronisation. All the pairs were at the same stage of the nesting period, and the synchronisation was not significantly related to the brood size. What drives this variation is interesting on its own and could reflect inter-pair variation in various traits that may be linked with parental performance. Examining such a variation may help to understand a general mechanism of the synchronisation. Importantly, high level of synchronisation in RW was associated with lower defecation rate in the nest. We assumed that synchronisation of the nest visits is driven by the partners’ cooperation. However, there is also a possibility that nest visit synchronisation is a demonstration of the sexual conflict over parental care (mutual control of the partners). If this is the case then our findings on decrease in defecation rate with increase of the synchronisation could be a cost of this conflict. It would not be the first time, when sexual conflict resulted in less efficient outcome of the breeding pair. As a matter of fact such a scenario has been already predicted by some theoretical models (Webb et al. 2002; Lessells and McNamara 2012) and has also been found to be the case in some empirical studies, although in the context of nest visit alternation. For example, the great tit (Parus major) parents alternated their visits to the nest and speed up their feeding rate after their partner had visited the chicks, but slow it down again once they had visited in turn (Johnstone et al. 2014). In such a context, low level of nest visit synchronisation in the RW could mitigate this negative effect, and counter-intuitively would suggest parent’s cooperation being a selective force. The parents avoiding a mutual control would maximise regularity in the feeding rate. Then, if there are no other forces favouring synchronisation of nests visits, parents meeting at the nest could simply be a coincidence.
Low level of nest-visit synchronisation observed among the RW pairs in the present study may partly be related to the breeding stage. We deliberately focused on that stage, to ensure both sexes are fully engaged in chick provisioning (females are not burden with extensive chick brooding). It is possible, however, that at this time the synchronisation, whatever purpose it serves for, it is not that much important any more. Both theoretical model (Lessells and McNamara 2012) as well as empirical studies (Leniowski and Węgrzyn 2018a; Baldan and Griggio 2019) have demonstrated that the level of synchronisation, if to maximise fitness offspring, is critical at early level of the reproduction. If the synchronisation is to better distribute the food among the chicks, then perhaps it is also much more important at the early stage, when chicks may be more sensitive to possible parental mistakes in food distribution. Then, if the synchronisation is to minimise the risk of the nest predation as in Leniowski and Węgrzyn (2018a), again it may be much more relevant at early stage, when chicks are more vulnerable to nest predation due to their inability to escape and survive outside the nest without parental brooding. Indeed, our data show that once chick reaches age of 8 days, their predation-related survival is rather high (94%). Finally, if the synchronisation is to control the partner’s behaviour and parental effort, perhaps this control may be the most important at the beginning of parental performance, when the level of partner commitment is not yet well established (Baldan and Griggio 2019).
Summing up, RW parents cooperate with each other raising together the common offspring. Both sexes exhibit the same effort in respect to brood size, and parental effort is correlated within the pair, which further suggests there are some interactions either between the parents and/or parents and environment that lead to the observed pattern. Similarly high parental effort seems to be beneficial for the offspring (better current condition of chicks) which suggests that cooperation may be a selective power shaping the parenting strategy. However, RW parents do not synchronise their feeding visits more often that it could be expected by chance, which is surprising given behaviour of other species, of a similar breeding ecology. The low level of parents’ synchronisation in the RW may be related to the late breeding stage that we considered in the presented study. Alternatively, it may be an adaptive parents’ performance, mitigating a negative effect of the synchronisation on the offspring fitness, as apparently the level of synchronisation is negatively related to the feeding rate in the nest.
Data availability
Data associated with the manuscript, including R-scripts are available at this link: https://osf.io/fwm6d/
References
Baldan D, Griggio M (2019) Pair coordination is related to later brood desertion in a provisioning songbird. Anim Behav 156:147–152. https://doi.org/10.1016/j.anbehav.2019.08.002
Baldan D, Ouyang JQ (2020) Urban resources limit pair coordination over offspring provisioning. Sci Rep 10:15888. https://doi.org/10.1038/s41598-020-72951-2
Baldan D, van Loon EE (2022) Songbird parents coordinate offspring provisioning at fine spatio-temporal scales. J Anim Ecol 91:1316–1326. https://doi.org/10.1111/1365-2656.13702
Borowiec M (1992) Breeding ethology and ecology of the reed warbler, Acrocephalus scirpaceus (Hermann, 1804) at Milicz, SW Poland. Acta Zool Cracov 35:315–350
Brown P, Davies M (1949) Reed warblers. Foy Publications Ltd, East Moseley, Surrey
Chase ID (1980) Cooperative and noncooperative behavior in animals. Am Nat 115:827–857. https://doi.org/10.1086/283603
Clutton-Brock TH, Vincent ACJ (1991) Sexual selection and the potential reproductive rate of males and females. Nature 351:58–60
Dewey M (2023) Package ‘metap’. Meta-analysis of significance values. R-package version 1.9. https://cran.r-project.org/web/packages/metap/index.html
Duckworth J (1990) Parental care in the reed warbler. Dissertation, University of Cambridge
Enns J, Williams TD (2022) Paying attention but not coordinating: parental care in European starlings, Sturnus vulgaris. Anim Behav 193:113–124. https://doi.org/10.1016/j.anbehav.2022.09.009
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330. https://doi.org/10.1111/2041-210X.12584
Ghalambor CK, Peluc SI, Martin TE (2013) Plasticity of parental care under the risk of predation: how much should parents reduce care? Biol Lett 9:20130154. https://doi.org/10.1098/rsbl.2013.0154
Griffith SC (2019) Cooperation and coordination in socially monogamous birds: moving away from a focus on sexual conflict. Front Ecol Evol 7:455. https://doi.org/10.3389/fevo.2019.00455
Halupka L, Halupka K, Klimczuk E, Sztwiertnia H (2014) Coping with shifting nest predation refuges by European reed warblers Acrocephalus scirpaceus. Plos One 9:e115456. https://doi.org/10.1371/journal.pone.0115456
Halupka L, Borowiec M, Neubauer G, Halupka K (2021) Fitness consequences of longer breeding seasons of a migratory passerine under changing climatic conditions. J Anim Ecol 90:1655–1665. https://doi.org/10.1111/1365-2656.13481
Hałupka L, Sztwiertnia H, Marczuk M, Dziachan A, Kosmowska A, Klimczuk E, Halupka K (2018) Ageing nestlings of the Reed Warbler Acrocephalus scirpaceus. Ringing Migr 33:1–9. https://doi.org/10.1080/03078698.2018.1546485
Houston A, Szekely T, McNamara J (2005) Conflict between parents over care. Trends Ecol Evol 20:33–38. https://doi.org/10.1016/j.tree.2004.10.008
Hua F, Sieving KE, Fletcher RJ, Wright CA (2014) Increased perception of predation risk to adults and offspring alters avian reproductive strategy and performance. Behav Ecol 25:509–519. https://doi.org/10.1093/beheco/aru017
Johnstone RA (2011) Load lightening and negotiation over offspring care in cooperative breeders. Behav Ecol 22:436–444. https://doi.org/10.1093/beheco/arq190
Johnstone RA, Hinde CA (2006) Negotiation over offspring care - how should parents respond to each other’s efforts? Behav Ecol 17:818–827. https://doi.org/10.1093/beheco/arl009
Johnstone RA, Savage JL (2019) Conditional cooperation and turn-taking in parental care. Front Ecol Evol 7:335. https://doi.org/10.3389/fevo.2019.00335
Johnstone RA, Manica A, Fayet AL, Caswell Stoddard M, Rodrigez-Girones MA, Hinde CA (2014) Reciprocity and conditional cooperation between great tit parents. Behav Ecol 25:216–222. https://doi.org/10.1093/beheco/art109
Karasov WH, Wright J (2002) Nestling digestive physiology and begging. Competition, cooperation and communication. In: Wright J, Leonard ML (eds) The evolution of begging. Kluwer Academic Publishers, Dordrecht, pp 199–221
Kilner RM (2001) A growth cost of begging in captive canary chicks. P Natl Acad Sci USA 98:11394–11398. https://doi.org/10.1073/pnas.191221798
Kokko H, Jennions MD (2008) Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948. https://doi.org/10.1111/j.1420-9101.2008.01540.x
Krebs EA, Cunningham RB, Donnelly CF (1999) Complex patterns of food allocation in asynchronously hatching broods of crimson rosellas. Anim Behav 57:753–763
Leniowski K, Węgrzyn E (2018a) Synchronisation of parental behaviours reduces the risk of nest predation in a socially monogamous passerine bird. Sci Rep 8:7385. https://doi.org/10.1038/s41598-018-25746-5
Leniowski K, Węgrzyn E (2018b) Equal division of parental care enhances nestling development in the Blackcap. Plos One 13:e0207757. https://doi.org/10.1371/journal.pone.0207757
Lessells CM, McNamara JM (2012) Sexual conflict over parental investment in repeated bouts: negotiation reduces overall care. Proc R Soc Lond B 279:1506–1514. https://doi.org/10.1098/rspb.2011.1690
Mariette MM, Griffith SC (2012) Nest visit synchrony is high and correlates with reproductive success in the wild Zebra finch Taeniopygia guttata. J Avian Biol 43:131–140. https://doi.org/10.1111/j.1600-048X.2012.05555.x
Mariette MM, Griffith SC (2015) The adaptive significance of provisioning and foraging coordination between breeding partners. Am Nat 185:270–280. https://doi.org/10.1086/679441
Martin TE, Briskie JV (2009) Predation on dependent offspring: a review of the consequences for mean expression and phenotypic plasticity in avian life history traits. Ann NY Acad Sci 1168:201–217. https://doi.org/10.1111/j.1749-6632.2009.04577.x
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956. https://doi.org/10.1111/j.1469-185X.2010.00141.x
Orłowski G, Hałupka L, Klimczuk E, Sztwiertnia H (2016a) Shell thinning due to embryo development in eggs of a small passerine bird. J Ornithol 157:565–572. https://doi.org/10.1007/s10336-015-1295-1
Orłowski G, Hałupka L, Pokorny P, Klimczuk E, Sztwiertnia H, Dobicki W (2016b) Variation in egg size, shell thickness, and metal and calcium content in eggshells and egg contents in relation to laying order and embryonic development in a small passerine bird. Auk 133:470–483. https://doi.org/10.1642/AUK-16-16.1
Quan R-c, Li H, Wang B, Goodale E (2015) The relationship between defecation and feeding in nestling birds: observational and experimental evidence. Front Zool 12:21. https://doi.org/10.1186/s12983-015-0116-y
Rooij EP, Griffith SC (2013) Synchronised provisioning at the nest: parental coordination over care in a socially monogamous species. PeerJ 1:e232. https://doi.org/10.7717/peerj.232
Schulze-Hagen K, Leisler B, Winkler H (1996) Breeding success and reproductive strategies of two Acrocephalus warblers. J Ornithol 137:181–192. https://doi.org/10.1007/BF01653633
Servedio MR, Powers JM, Lande R, Price TD (2019) Evolution of sexual cooperation from sexual conflict. P Natl Acad Sci USA 116:23225–23231. https://doi.org/10.1073/pnas.1904138116
Shen SF, Chen HC, Vehrencamp SL, Yuan HW (2010) Group provisioning limits sharing conflict among nestlings in joint-nesting Taiwan yuhinas. Biol Lett 6:318–321. https://doi.org/10.1098/rsbl.2009.0909
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639–1644. https://doi.org/10.1111/2041-210X.12797
Svensson L (1992) Identification guide to European passerines, 4th edn. British Trust for Ornithology, Stockholm
Trivers R (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man, 1871–1971. Aldine, Chicago, pp 136–179
Webb JN, Székely T, Houston AI, McNamara JM (2002) A theoretical analysis of the energetic costs and consequences of parental care decisions. Phil Trans R Soc B 357:331–340. https://doi.org/10.1098/rstb.2001.0934
Wojczulanis-Jakubas K (2021) Being the winner is being the loser when playing a parental tug-of-war – a new framework on stability of biparental care. Front Ecol Evol 9:763075. https://doi.org/10.3389/fevo.2021.763075
Wojczulanis-Jakubas K, Araya-Salas M, Jakubas D (2018) Seabird parents provision their chick in a coordinated manner. Plos One 13:e0189969. https://doi.org/10.1371/journal.pone.0189969
Acknowledgements
We thank to Monika Czuchra, Beata Czyż, Weronika Kasprzak, Garbiela Krogulec, Aneta Rybińska, and Zofia Zalejska for their help in the field and Yuki Brooknievskaya for her inspirational spirit and overall support (and patience) during conceptualisation of the project and writing the manuscript. Thanks also go to anonymous reviewers for their valuable comments that helped to improve the manuscript.
Funding
The study was supported by funds of University of Wrocław.
Author information
Authors and Affiliations
Contributions
Conceptualisation: KW-J and LH; methodology: LH; fieldwork: LH and JP; video analysis: KW-J, JP, and AG; data analysis: KW-J and AG; data curation: LH; initial draft: KW-J; editing: all authors.
Corresponding author
Ethics declarations
Ethical approval
The study was carried out with some handling (colour ringing) and video recording of wild birds in their nest site area. This was performed with the highest care, following the ARRIVE guideline and relevant permits from Regional Inspectorate of Environmental Protection (WPN.6205.36.2019.MR2) and Local Ethical Committee (20/2019). All the handled birds were released after 3 min of handling and show no sign of distress. Also video recording did not disturb natural behaviours, and all the recorded individuals behave without apparent signs of any distress.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by M. Leonard
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wojczulanis-Jakubas, K., Płóciennik, J., Guinebretiere, A. et al. Cooperative parental performance at chick provisioning in a small passerine, the Reed Warbler Acrocephalus scirpaceus. Behav Ecol Sociobiol 77, 123 (2023). https://doi.org/10.1007/s00265-023-03397-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03397-5