Abstract
Mate search is challenging for solitary species. Trails represent a particularly potent, target-oriented means for finding mates, as trail-following increases encounter rates between individuals compared to random search. Embedding directionality information into the trail allows individuals to follow trails correctly to the source. Yet, directionality remains poorly understood. Spiders deposit trails during locomotion consisting of silk lines and substrate-borne chemicals. We conducted multiple experiments to test whether female silk trails convey directionality information, whether directionality is chemically or structurally encoded and depends on female phenotype. We also examined whether males interact with silk in a way that suggests information gathering. We exposed males of the cursorial spider Pisaura mirabilis to female trails deposited unidirectionally and scored their trail-following direction (i.e., same as or opposite to the females’). Tests were repeated after washing trails with a solvent to remove putative chemicals and by sourcing silk from females of different feeding regimes. While we found little evidence for male directional trail-following, we did find that unwashed trails were more likely to be followed than washed trails. Similarly, trails of relatively larger females were more likely to be followed correctly than those of smaller females. Males extensively probed and pulled on silk lines with their appendages, suggesting the gathering of chemical and tactile information. Taken together, results suggest that directional trail-following is selected only under specific contexts in this species. Chemical attributes of trails may convey information on female quality, with directional trail-following reflecting male mate choice in a system characterized by costly male nuptial gifts.
Significance statement
In the context of male mate search, following conspecific trails increases the chances of encountering a mating partner, especially if trails provide information about the direction the conspecific went. Yet, trail directionality remains poorly understood. Female spiders deposit silk trails as they walk. We overall show that males follow trails directionally only under a specific context. Males were more likely to follow correctly when trails were unmanipulated (compared to being washed with solvents to remove chemicals) and when they were deposited by relatively larger females (compared to smaller ones). Chemical attributes of trails may potentially indicate directionality, while decoding movement direction from trails of larger females may reflect male preferences for females of higher reproductive value.
Similar content being viewed by others
Introduction
Finding a mating partner is essential for animal reproduction and potentially challenging for solitary species with widely dispersed individuals in the population (Bell 1990). Animals rely on multiple cues (i.e., incidental sources of information) and signals (i.e., information evolved to be communicated and to change the behavior of the receiver) during mate search (Bradbury and Vehrencamp 2009; Stevens 2013). Long distances between senders and receivers are often covered through auditory signals, as bird songs (Hinde 1971; Todt and Naguib 2000) and cricket calls (Forrest 1982), or by producing long-range airborne chemicals, as odor plumes of female moths (Butt and Hathaway 1966; Weatherston and Percy 1977). Vibrational (Rovner and Barth 1981; Virant-Doberlet and Cokl 2004) and visual signals (Rutowski et al. 2001) are instead generally used for mid- to close-range mate localization. Animals can also use trails for mate search that are deposited on the substrate and accessible to conspecifics. Such trails can consist of chemical information, such as pheromones, which are chemicals emitted to alter a conspecific’s behavior (Karlson and Lüscher 1959). Trails can also be created through physical alterations of the environment, for example, by cutting trails into the vegetation for eased locomotion as done by leaf-cutter ants (e.g., Howard 2001). Trails represent perhaps the most potent and target-oriented means for finding mates and food sources across taxa (e.g., social insects Wilson 1962; Jeanne 1981; Nieh et al. 2004; Jarau 2009; Bordereau and Pasteels 2010; Czaczkes et al. 2015), reptiles (Gehlbach et al. 1971; Cooper and Vitt 1986), mollusks (Cook and Cook 1975; Ng et al. 2013; Vong et al. 2019), and mammals (Jamon 1994; Harmsen et al. 2010)). By adding specific information to a trail (e.g., pheromones), the trail producer can inform the following conspecific about its sex, mating status, or body condition, allowing both advertisement of the producer and assessment by the trail follower (e.g., Gehlbach et al. 1971; Edwards and Davies 2002; O’Donnell et al. 2004; Baruffaldi et al. 2010). Apart from extrapolating information on the phenotypic characteristics of its producer, when encountering a trail, an individual has to choose a direction in which to proceed. While a correct choice results in a high probability of finding a mate, a wrong choice takes the animal further away from its goal. Thus, any aid to the individual in determining the direction to follow, such as polarized trails embedded with directionality information, provides benefits (Cook and Cook 1975; Tietjen 1977; Rosengren and Fortelius 1987; Nieh et al. 2004). Embedded trail directionality appears to be rare as it has been described in only a few species (e.g., Gehlbach et al. 1971; Tietjen and Rovner 1980; Jackson et al. 2004; Nieh et al. 2004; Ng et al. 2013), and its respective encryption in the trail is understood in even fewer cases (Ford and Low 1984; Jackson et al. 2004). For example, snakes can encrypt trail polarity by sequentially touching a specific surface of ambient objects (e.g., anterolateral) with pheromone-secreting glands during movements (Ford and Low 1984). Ants instead create non-random bifurcation angles in their trail networks, allowing the polarity of a trail to be read at every bifurcation (Jackson et al. 2004). The occurrence of directionality information in trails may reflect variations in species communication modalities, spatial distributions, population densities, and life history. Trail directionality is, for example, not expected when the costs of trail deposition including embedding of directionality information, in terms of energetic costs (i.e., production and/or secretion of substances) or ecological costs (i.e., increased visibility to predators), override the benefits of finding the trail producer. The limited evidence for trail directionality may, however, also result from a small number of empirical studies focusing on few taxonomical groups (e.g., molluscan mucous trails and pheromone trails of ants) (Rosengren and Fortelius 1987; Jackson et al. 2004; Ng et al. 2013; Czaczkes et al. 2015), leading to bias in the literature. Hence, overall trail directionality remains poorly understood.
Spider silk lines, consisting of proteinaceous silk fibers and other substances (i.e., pheromones) (Foelix 2010), are a medium for mate localization. Females of many web-building spiders add airborne pheromones to their webs to attract mating partners from a distance (Witt 1975; Ross and Smith 1979; Jackson 1987; Chinta et al. 2010; Fischer et al. 2021). Direct contact with the female’s web often induces explorative behavior and/or positive chemotaxis in the male, further aiding mate search (Suter and Renkes 1982; Watson 1986; Suter et al. 1987). During movements, web-less cursorial spiders release long silk lines (i.e., draglines) with a stabilizing function (van der Kraan and Richter 1970). Chemical and tactile properties of draglines are known to stimulate male sexual behaviors (Jackson 1987; Barth 1993; Chinta et al. 2010; Beyer et al. 2018; Eberhard et al. 2021) and to induce random mate search (Hegdekar and Dondale 1969; Yoshida and Suzuki 1981; Taylor 1998). Draglines represent trails, as males that follow these silk lines can greatly increase their chances of finding a mate compared to random search (Tietjen 1977; Anderson and Morse 2001; Bell and Roberts 2016; Scott et al. 2019). Yet, trail directionality has been investigated in a handful of spiders (Dijkstra 1976; Tietjen 1977; Anderson and Morse 2001; Bell and Roberts 2016) and has only been found in two species of wolf spiders, Lycosa rabida and L. punctulata (Tietjen 1977). The encryption of directional information in spider trails has yet to be decoded. While trail-following is hypothesized to be induced chemically, trail directionality itself is thought to be encoded structurally in spider silk, possibly with the help of so-called attachment discs, silken structures that anchor the silk line to the substrate (Apstein 1899; Dijkstra 1976). Spiders might be able to assess differences in silk tension in the area surrounding the discs (Wolff and Herberstein 2017; Wolff et al. 2021) by using their legs or pedipalps (i.e., a modified pair of appendages), which additionally possess specialized chemo-sensing structures (Foelix 2010; Müller et al. 2020). Indeed, trail-following is described as being accompanied by extensive probing of silk threads through the male’s pedipalps (Tietjen 1977; Tietjen and Rovner 1980).
With their solitary lifestyle and the almost omnipresent use of silk lines for reciprocal communication between the sexes (Beyer et al. 2018; Eberhard et al. 2021), cursorial spiders, such as the nursery-web spider Pisaura mirabilis, represent an extremely promising system for investigating directionality in silk trails. This species is well known for the male’s food donations to the female (i.e., nuptial gifts) that are crucial for mate acceptance and mating (Nitzsche 1988; Stålhandske 2001; Albo et al. 2011; Ghislandi et al. 2017). Draglines of females are embedded with tactile chemicals (Beyer et al. 2018) that induce male courtship behavior (Eberhard et al. 2021) and silk-wrapping of nuptial gifts (Bilde et al. 2007; Albo et al. 2011; Ghislandi et al. 2017; Magris and Tuni 2019). These also communicate the female developmental state (Eberhard et al. 2021) and body condition (Beyer et al. 2023b) to the male. During the mating season, males and females actively move around the vegetation (Ghislandi et al. 2018) and would benefit from depositing silk trails with directionality information to increase their encounter rates. Due to the costs of gift construction associated with lost foraging opportunities, silk production, and carrying costs (Lang 1996; Albo et al. 2011; Prokop and Okrouhlík 2021), males are particularly expected to exploit information left in the environment by female trails to orientate and locate females during mate search. From the female perspective, being found by a male not only ensures multiple matings but also leads to foraging benefits due to the nutritional value of nuptial gifts (Tuni et al. 2013; Toft and Albo 2015). Embedding directional information in trails may also likely depend on the female’s phenotype, as in spiders, silk lines structural properties such as density and size (Vollrath 1999) as well as chemical signaling bound to silk (Weiss and Schneider 2022a, b), may depend on the releasers’ body mass. Although a formal test for directionality is lacking, when given a choice, P. mirabilis males preferentially follow silk trails deposited by females in high body condition, compared to those of low-condition females (Beyer et al. 2023b). These findings may suggest chemical and/or structural differences in silk trails that trigger a differential response in mate-searching males. Male trail-following may ultimately reflect mate choice decisions, with female body mass, size, and condition (i.e., a trait reflecting both body mass and size) indicating higher fecundity in arthropods (Bonduriansky 2001; Leather 2018) including spiders (Danielson-Francois et al. 2002; Johnson et al. 2014), and hence females of higher reproductive value. Not least, trail-following may also be affected by the male’s own individual phenotypic characteristics (body condition, mass, or size), known to affect the strength of choice (Pollo et al. 2022), or on those of both interacting sexes.
Here, we hypothesize that (i) female silk trails provide directional information to males and that such information is either chemically or structurally mediated; (ii) silk trails provide information on female phenotypic characteristics (body mass, size, and condition) that affect directional trail-following in males; and (iii) males assess silk line properties (e.g., chemicals and/or silk tension) using their appendages. Hence, we conducted three experiments. In the first experiment, we placed males in the middle of a silk trail unidirectionally deposited by a female and scored whether males moved in the same or opposite direction to female movements during trail deposition. In this experiment, we also included a treatment, where putative chemicals of trails were removed by washing with a solvent. If trails provide directional information, males are expected to move in the same direction the silk was deposited by females. If this information is solely chemically transmitted, directional information should be lost when trails are washed, whereas if it is mediated by silk structure, males should move in the direction of the female regardless of trail washing. In a second experiment, we manipulated female body condition through differential feeding regimes and tested whether male movements in the same or opposite direction to female movements during trail deposition depend on female body mass, condition, and size. If trail deposition reflects female phenotypic characteristics, males should be more likely to move in the direction of females with high-trait values, representing more fecund mating partners. Finally, in a third experiment, we analyzed high-speed video recordings of males in contact with female trails surrounding the area of silken attachment discs. If males perform leg and pedipalp movements on silk lines, these might indicate male sensory assessment of trails, such as silk tension assessment by pulling and chemical assessment by probing silk.
Experiment 1. Do female silk trails convey directional information to males, and is this chemically or structurally mediated?
Methods – experiment 1
Animal collection and rearing
Juvenile and subadult Pisaura mirabilis of both sexes (n = 19 females, n = 31 males) were collected in Planegg-Martinsried near Munich (Germany) during spring 2021. They were brought to the lab of the Ludwig-Maximilians-University of Munich, where they were individually placed into transparent plastic vials (9 cm height, 5 cm diameter) that were covered with foam lids. Animals were reared at room temperature (approx. 23 °C) and under a natural photoperiod (15 h light: 9 h dark). To maintain high humidity, vials contained moss that was sprayed with water on feeding days. Spiders were fed three times a week, depending on their age, using either 15 fruit flies (Drosophila melanogaster) for juveniles, or two cricket nymphs (Gryllus bimaculatus) or two house flies (Musca domestica) for (sub-)adults. Prey type for (sub-)adult animals varied due to logistic reasons. To minimize diet-dependent variation in the chemical composition of silk (Craig et al. 2000; Tso et al. 2005) and silk-borne chemicals (Henneken et al. 2015, 2017), each spider was tested on a single day (reduction of within-individual variation) and, on a feeding day, in which all (sub-)adult spiders received the same prey type (reduction of between-individual variation). Vials were inspected daily for molted exoskeletons to control for spider maturation to adulthood. Individuals were tested approximately 2 weeks after their molt to adulthood and were of similar age to minimize putative age-dependent variation in sexual signaling known in other spider species (e.g., Baruffaldi and Costa 2010; Cory and Schneider 2016). All animals remained unmated through the course of the experiments.
Experimental setup
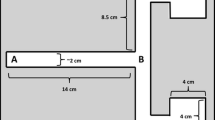
To test for directional trail-following, we used a testing corridor (60 cm length × 12 cm height × 7 cm width) (Fig. 1) made of polystyrene foam, with all internal surfaces covered with brown parcel tape (Tesapack 64014) to ease cleaning. Two holes (5 cm diameter) were cut in both side walls of the device for the female to enter and exit, and one in the middle of one long side (center hole) of the device through which the focal male could enter. Each hole could be closed with a fitting foam lid to prevent spiders from exiting the device prematurely.
Schematic representation of the testing corridor with the male spider encountering the single unidirectionally deposited female silk trail (dashed line) via the center hole in the long side of the device and moving either in the same direction as the female (indicated by the arrow at the end of the silk line) or the opposite toward one of the exit holes
Female trails were obtained by allowing sexually mature females to enter the testing device on one side and move freely to the opposite end without changing direction, hence reliably depositing a unidirectional single trail consisting of silk and possibly female body odor (experiment 1a). In cases where the female changed direction or stopped moving, the female was removed, the device cleaned with ethanol (EtOH; extra pure, Carl Roth, Germany) and dried using a cotton cloth before repeating the trial. Once the female left the device through the exit hole on the opposite side to where it had entered, it was returned to its housing vial. Female silk lines were visible to the observer. The direction of the female’s movement (left to right or vice versa) was randomized before each trial to account for directional biases. A male, not carrying a nuptial gift, was introduced through the center hole of the device immediately after female removal, and his movements (left to right or vice versa) were observed. A trial was considered completed when the male reached one of the holes for exiting the device.
We included a washed treatment (experiment 1b), where, after the female had walked through the device, the inside of the device was sprayed with 96% EtOH to remove putative chemicals on the silk and on the device’s substrate (left from the female’s body). Ethanol was chosen as it is able to dissolve both polar and (weakly) non-polar substances due to its polar (hydroxyl (OH)) and non-polar (ethyl (C2H5)) groups. While it might not be able to remove all silk-borne chemicals, especially complex lipids such as long-chained glyceryl ethers that are found in/on silk (Chinta et al. 2010; Schulz 2013; Gerbaulet et al. 2022), we would still expect to dissolve a variety of (organic) compounds known to function as spider pheromones such as some alkanes found in Araneus diadematus (Schulz 2013; Fischer 2019). After spraying the ethanol, the device was left to dry completely (approx. 5–10 min) before the male was introduced into it. We also carried out an additional control treatment (experiment 1c) that consisted of allowing males to enter empty cleaned devices to investigate whether male directional decisions are subject to directional biases, with males preferably choosing a specific direction in which to proceed. This control treatment was repeated three times for each male. To test for potential effects of washing treatments on silk structure, we inspected draglines washed with polar (water) or non-polar (pentane) solvents using scanning electron microscopy (experiment 1d), and, as reported in the Online resource, results suggest no visible structural differences between washed and unwashed draglines, confirming results of a previous study (Shao and Vollrath 1999) (Supplementary Figs. S1 and S2 and Table S1).
Each male (n = 31) was tested five times using an unwashed trail (experiment 1a), a washed trail (experiment 1b), and three times no-trail controls (experiment 1c) in a randomized order and on a single day. Each male was exposed to silk sourced from the same female during unwashed and washed-trail treatments. Due to a low available number of females (n = 19) of similar age range (i.e., 13–16 days from final molt), some females were used with multiple males. Each trial was video recorded using a webcam (Logitech HD Pro Webcam C920) fixed at a distance of 40 cm over the testing device and connected to a laptop. After each trial, males were returned to their housing vials, and the device was cleaned using EtOH and dried before re-use. Body mass of males and females was measured to the nearest 0.01 g using a digital scale (Kern PKT, Kern & Sohn GmbH, Germany) before testing (hence, if present, nuptial gifts were gently removed from males using forceps), and prosoma width was measured as a proxy for spider body size to the nearest 0.01 mm using electronic calipers (Aerospace, China) once experiments were completed.
Scoring of male trail-following
Behavioral parameters were scored from the videos using the event-logging software BORIS (v7.10.5) (Friard and Gamba 2016). We scored which end of the device was reached by the male (same as or opposite to female movements) and measured the total amount of time the male spent in the device, from entering the device to touching one of the exit holes on the sides, as an indicator of the duration of male assessment of cues in the environment. We additionally noted the occurrence of directional turns, defined as a 180° turn of the animal (yes/no) before the male touched one of the exit holes of the device to explore their function as potential indicators of accessibility of directional information. To minimize observer bias, blinded methods were used when all behavioral data were analyzed by keeping the observer naïve to the identities and treatments of the spiders observed in the videos.
Statistical analyses
To test the hypothesis that female silk trails provide directional information to males (experiment 1a), we conducted a binomial test, testing the proportion of male choices in the correct direction (i.e., same direction as the silk was deposited by the female).
To test whether directional information is chemically mediated and to account for the data structure involving multiple uses of animals, we fitted a binomial generalized linear mixed-effects model (GLMM), including male movement in the correct direction as response variable, treatment (unwashed and washed trails from experiment 1a and b) and testing order (to account for increasing male experience with the testing device) as fixed effects. To account for multiple use of spiders (i.e., to avoid pseudo-replication), spider identities of each sex were added as random effects. Finally, we conducted binomial tests to test for male biases in moving consistently in one specific direction (experiment 1c) both over the course of the experiment and on specific days (see Online resource).
In addition to our main questions described above, we conducted four additional explorative models to test whether (i) males that spent more time assessing the environment and/or performing directional turns are more likely to follow trails correctly (i.e., in the direction deposited by the female), (ii) female phenotypic characteristics (body mass, condition, and size) influence male directional trail-following, with males following correctly high-trait females and effects being lower in the washed treatment due to removal of chemically encoded information on female phenotype, (iii) male phenotypic characteristics (body mass, condition, and size), and (iv) relative differences between female and male phenotypic characteristics influence male directional trail-following, with males following correctly females with relatively higher relative trait differences. Body condition was calculated using a residual body condition index, defined as the residuals of a linear regression of body mass on size (i.e., prosoma width) (Jakob et al. 1996). Detailed description of statistical models is reported in the Online resource.
All numeric variables were grand-mean-centered by subtracting each value from the variable’s overall mean to ease biological interpretation and standardized using the standard deviation of the variable. Statistical analyses were performed using R (version 4.1.1, R Core Team 2021). Binomial generalized linear mixed-effect models (GLMMs) were applied using the “stan_glmer” function, which included the simulation of posterior distributions based on 4000 iterations (package “rstanarm”) (Goodrich et al. 2020). Model fit was visually assessed based on the model’s fitted values (goodness of fit graph) and the distribution of residuals. The statistical significance of fixed effects was inferred from the Bayesian 95% credible intervals (CI) associated with the mean parameter estimate (β) with effects being considered significant in the frequentist’s sense when the 95% CIs did not overlap zero (Nakagawa and Cuthill 2007).
Results – experiment 1
Within each treatment, males did not display a significant preference for choosing the same direction as the female (binomial test: unwashed treatment: p = 0.28, 61%, 95% – CI: 42–78%, 19 out of 31; washed treatment p = 0.15, 35%, 95% CI, 19–55%, 11 out of 31) (Fig. 2). Males chose the same direction as the female that deposited the trail significantly more often when the trail was unwashed in comparison to when it was washed with EtOH (Table 1). Testing order did not influence the response significantly (Table 1).
Likelihood of males following the trail in the same direction as deposited by the female when trails are unwashed (experiment 1a) and washed with solvents (experiment 1b). The dashed line indicates random choice. Error bars represent 95% credible intervals of the binomial test. Numbers within bars = number of males following in female direction out of all males within treatment. Directional trail-following was significantly higher in unwashed trails
Males in repeated control (no-trail) treatments did not show directional bias over the course of the experiment: Individual males did not preferentially choose the same direction as opposed to varying directions during their three no-trail trials (within-individual bias; binomial test: p = 0.28, 39%, 95% – CI: 22–58%, 12 out of 31), and all males did not choose a specific direction (right or left) in the testing device when exposed to female trails (between individual bias; binomial test: p = 0.47, 46%, 95% – CI: 35–56%, 42 out of 92). Model results were retained after excluding a single biased day from the data (see Supplementary Table S2).
When exploring other factors, such as male behaviors during the trial (time spent in the device, occurrence of turns) and male and female phenotypic traits (body mass, condition, and size) that may affect male directional decisions, we found the following: (i) the total time spent in the device and 180° directional turns did not explain variation in directional male trail-following (Supplementary Figs. S3 and S4 and Table S3); (ii) males were more likely to follow trails in the same direction as the female laid them when the female had high body mass and condition (Supplementary Table S4 and Fig. S5) but not large size (Supplementary Table S4). The significant effect of the washing treatment was retained, yet the effect of female body mass was significantly smaller in the washed than in the unwashed treatment; (iii) male body mass, condition, and size did not affect the directional trail-following (Supplementary Table S5), (iv) nor did the relative difference in mass or size between the sexes (Supplementary Tables S6 and S7).
Experiment 2. Do silk trails provide information on female phenotypic characteristics (body mass, size, and condition) that affect directional trail-following in males?
Methods– experiment 2
Animal collection and rearing
Juvenile and subadult P. mirabilis of both sexes (n = 153) were collected during spring and autumn 2022 in the same locality as described for experiment 1. For animals caught in spring, room temperature and natural photoperiod were, on average, 25 °C and 15 h light: 9 h dark. To ensure a comparable room temperature for animals reared in autumn/winter, vials were placed on heating mats (approx. 23 °C). The natural photoperiod for these animals was 10 h light: 14 h dark. The seasonal differences between the two cohorts of spiders were statistically addressed (see below).
To obtain females varying in body mass, we applied a differential feeding treatment to which a randomly chosen subset of females was assigned to as soon as they reached adulthood for a total of 2 weeks. The low-fed regime was implemented by providing spiders once a week with one housefly (n = 48 females), and the well-fed feeding regime by providing 1 fly three times a week (n = 53 females). Well-fed females had a significantly higher body mass (two-sample t-test: t = − 8.20, d.f. = 112.76, p < 0.001; mean mass ± s.e. in mg: well-fed: 116.00 ± 2.56, n = 58; low-fed: 85.79 ± 2.65, n = 57) and body condition than low-fed females (two-sample t-test: t = − 7.18, d.f. = 111.48, p < 0.001; mean body condition residual index ± s.e.: well-fed: 12.46 ± 2.47, n = 58; low-fed: − 11.17 ± 2.17, n = 57). In autumn, well-fed females were also significantly larger in size than low-fed females, leading to overall larger well-fed females (two-sample t-test: t = − 2.42, d.f. = 106.90, p = 0.02; mean size ± s.e. in mm: well-fed: 3.59 ± 0.04, n = 58; low-fed: 3.48 ± 0.03, n = 57).
Experimental setup
To test for male directional trail-following of trails from high-fed (experiment 2a) and low-fed females (experiment 2b), we used the testing corridor and experimental approach previously described (Fig. 1). As the time spent in the device for trail deposition varied greatly between females, we measured and standardized the time spent in the device in a subset of females (n = 35) to control for possible variation in trail deposition (e.g., quantity of deposited silk or putative chemicals): If a female did not exit the device after 60 s, a paintbrush was tapped against the top of a side wall behind the female’s position to induce forward movement. We additionally carried out a single-run control treatment in an empty device (experiment 2c), testing males without female silk trails as described for experiment 1.
Each male (n = 52) was tested three times using the trail of a well-fed female (experiment 2a), of a low-fed female (experiment 2b), and a no-trail control (experiment 2c) in a randomized order and on a single day. Due to a low available number of females of a similar age range of 13–16 days from final molt, some females (n = 13) were used with multiple males. Each trial was video recorded using a webcam (Logitech HD Pro Webcam C920 or Logitech BRIO 4 K) fixed at a distance of 40 cm over the testing device and connected to a laptop. After each trial, males were returned to their housing vials, and the device was cleaned using 96% EtOH and dried before re-use. Body mass and size of males and females were measured as described in experiment 1.
Scoring of male trail-following
We scored which end of the device was reached by the male (same as or opposite to female) either from the videos using the event-logging software BORIS (v7.10.5) (in spring) or by direct observations (in autumn). As for experiment 1, observer bias was minimized using blinded methods when all behavioral data were analyzed.
Statistical analyses
As done in experiment 1, we first used the data to test the hypothesis that female silk trails provide directional information to males by conducting a binomial test within each treatment (experiments 2a and 2b).
To test the hypothesis that male trail-following is influenced by female phenotypic traits (body mass, size, and condition) (experiments 2a and 2b), with males being more likely to move in the direction the trail was deposited in the case of females with high-trait values representing more fecund partners, we fitted three distinct models with each of the female traits (either body mass, body size, or residual body condition index) and testing order as fixed effects. Spider identities of each sex were added as random effects to account for repeated measurements. As the season of experiment conduction (spring vs. autumn) did not explain variation in the data (see Supplementary Table S8), we removed it from the model simulations.
To test whether male phenotypic traits (male body mass, condition, and size) affect male movements on trails of high-fed and low-fed females, we expanded the models described above by fitting three distinct models, each with one of the male traits (either body mass, body size, or residual body condition index). To account for the phenotypic effects of both sexes, we repeated these models including the relative trait difference, calculated as the relative difference between male and female body mass and size (female value divided by male value). Values of the variable’s body mass, size, condition, and trial number were grand-mean-centered by subtracting each value from the variable’s overall mean and standardized using the standard deviation of the variable to ease biological interpretation of the model output.
Finally, we investigated male directional bias over the course of the experiment and on specific days (experiment 3c) by using a binomial test to compare the number of males choosing each direction during individual experimental days. As a single experimental day showed male directional bias (binomial test: p = 0.07, 88%, 95% – CI: 47–100%, 7 out of 8), analyses were repeated with a dataset excluding this biased day.
Statistical analyses were performed using R (version 4.1.1) and as described for experiment 1.
Results – experiment 2
Within each feeding treatment, males did not display a significant preference for choosing the same direction as the females (binomial test: well-fed: p = 0.36, 43%, 95% – CI: 30–57%, 25 out of 58 trials; low-fed: p = 1.00, 51%, 95% – CI: 37–64%, 29 out of 57 trials). Testing order did not significantly affect the response (Table 2). Males did not significantly differ in the likelihood of following trails in the same direction deposited by a high-mass (i.e., well-fed) compared to a low-mass (i.e., low-fed) female (Table 2 model a), nor by larger females or females in higher body condition (Table 2 models b and c).
Male likelihood to follow in female direction was not significantly influenced by either male body mass, size, or condition (Supplementary Table S9). While male likelihood to follow in the female direction was also not significantly influenced by the relative mass differences between the sexes, males were significantly more likely to follow in the female direction if the female was of relatively larger size than the male (Fig. 3, Table 3). This significant effect was also found for data collected in spring, but not in autumn (Supplementary Table S11).
The effect of the relative difference in body size between males and females on the male’s probability to follow trails in the same direction laid by females in experiment 2 (n = 115), with values > 1 indicating relatively larger females and values < 1 indicating relatively smaller females compared to male size. Males are significantly more likely to follow females that are relatively larger than they are. The dashed line represents the regression line, gray areas are the 95% credible intervals, and circles are the relative body size difference of the individual male and female pairings in the trials
The control (no-trail) treatment (experiment 2c) showed no directional male bias over the course of the experiment: Individual males did not preferentially choose a specific direction (right or left) in the testing device when exposed to female trails (binomial test: p = 0.16, 60%, 95% CI: 46–72%, 36 out of 60 for the left direction in the device). When excluding the biased day from the data and repeating the model simulations, results were retained (see Supplementary Table S12).
Experiment 3. Do male spiders interact with silk in a way that suggests information gathering?
Methods– experiment3
Animal collection and rearing
In spring 2021, juvenile and subadult Pisaura mirabilis of both sexes were collected in Greifswald (Mecklenburg-Vorpommern, Germany). They were brought to the lab of Greifswald University, where they were individually placed into transparent plastic vials (9 cm height, 5 cm diameter), covered with foam lids. Animals were reared at room temperature (approx. 23 °C) and under artificial light (three UV lamps, Exo Terra Repti Glo 10.0, 10% UV-B, 33% UV-A, 20 Watts) set to natural photoperiod. To maintain high humidity, vials had their bottom removed and were placed upside down in water-filled trays. Spiders were fed twice a week using either a common green bottle fly (Lucilia sericata) or 2 small crickets (Acheta domesticus, Gryllus assimilis), with spiders receiving the same prey type on a respective feeding day. To accelerate development, animals were placed inside a climate chamber for seven days (25 °C, 67% humidity).
Experimental setup
To study male movements on silk trails, we used a walking corridor (30 cm length × 5 cm width × 7 cm height) made of transparent acetate sheets. Female trails were obtained by letting a female walk unidirectionally through the walking corridor. To ensure the deposition of attachment discs, the corridor was tilted by 40°, resulting in the female slightly slipping while walking, inducing disc deposition to secure itself. After the female reached the end of the corridor, it was returned to its housing vial and the male was introduced to the starting point of the trail. A total of 11 males were tested multiple times (up to 5) using silk lines sourced from 9 females (total trial number n = 22).
Trials were video recorded using a manually post-triggered high-speed camera (MIRO LC 320S), connected to a laptop, with 100 mm Macro (Canon) fixed at a distance of 25 cm below the transparent runway, and the field of view of the camera was set to the area surrounding attachment discs (n = 20). In two additional trials, the camera was fixed above the runway, with the field of view set to the beginning of the silk thread. The video resolution was 250 frames per second. To ensure continuous illumination during the recording, two direct current flashlights (Heider CFX Super Power) were fixed at 10 cm distance below and next to the device at 120° and 40°, respectively. Spiders did not appear to be disturbed by these lights, and no noticeable behavioral changes were observed.
Scoring of male movement
Every video (n = 22) contained 2 real-time seconds worth of high-speed material, resulting in recordings of approximately 90 s duration. One video was excluded from further analyses due to the male not moving (total number of videos n = 21). These were scored manually by one observer (MB). Silk lines and attachment discs were visible in the videos. Movements of male appendages (legs and pedipalps) with respect to silk lines and attachment discs were observed to determine if and how males make physical contact with the silk.
This data was used to thoroughly describe the probing behavior of males when coming in contact with female dragline silk; no statistical analyses followed.
Results – experiment 3
We were able to detect three distinct male behaviors (i) in 91% of the trials (19 out of 21) males tapped the substratum with the tips of their pedipalps; (ii) from those trials in which males came into direct contact with a silk line (n = 19), in 42% of the trials (8 out of 19) males pulled on the silk line, guiding it either toward their body (Fig. 4a) or their pedipalps (Fig. 4b), using the tarsal claws of one of the 1st, 2nd, or 3rd pair of legs; (iii) silk lines were also commonly probed by males (16 out of 19 trials; 84%) by sliding along parts of it using the tips of one of the 1st pair of legs (7 out of 16 trials; 44%), or by placing the silk line on the ventral side of one of the pedipalps (pedipalpal cymbium) and guiding the silk line along it (15 out of 16; 94%). This was observed both from ventral and dorsal views of the animals (Fig. 4c,d). In none of the video recordings, males made direct physical contact with the attachment discs.
Video stills showing a male P. mirabilis filmed either from below (a–c) or above the transparent runway (d): (a) pulling on a silk thread with its first front leg (light gray arrow), (b) pulling on a silk thread with its front leg and a pedipalp (light gray arrows) while gliding along the line with the remaining pedipalp (dark gray arrow), (c) probing of a silk thread with a pedipalp in ventral view (light gray arrow), and (d) dorsal view of probing of a silk thread with a pedipalp (light gray arrow)
Videos of the above-mentioned behaviors can be viewed on Figshare (Beyer et al. 2023a).
Discussion
In this study, we investigated whether trails, consisting of silk lines and/or body odor, of females of the hunting spider Pisaura mirabilis carry directionality information that aids male mate search. We further examined whether directionality is chemically or structurally encoded and depends on female phenotypic characteristics (body mass, condition, and size). We found that, overall, males did not directionally follow female trails. However, these results varied in different experiments. Males were more likely to follow trails correctly when they were unwashed compared to when they were washed with a solvent to remove putative chemicals. Similarly, males were more likely to correctly follow trails when they were deposited by females that were relatively larger in size. We also examined whether males interact with silk in a way that suggests information gathering and, indeed, describe extensive probing and pulling on silk lines with their appendages. Taken together, while our findings on male trail-following behavior provide no overwhelming evidence for directional trail-following, our results that males are more likely to follow non-washed trails correctly and follow trails of relatively larger females lead us to conclude that directional trail-following might be present in this species, but facultative and fragile.
Contrasting to our expectations, Pisaura mirabilis males did not generally follow trails in the direction they were deposited by females. One possible explanation is that potential costs of encoding directionality information in a silk trail, such as production/secretion of directionality-inducing chemicals or structural components, or increased predation or parasitism risk (van Baarlen et al. 1996; Wignall and Taylor 2009; Fei et al. 2023), may outweigh the benefits of eased mate finding. Alternatively, directionality information in female trails is not necessary in this species. Despite not being territorial, spiders occur in relatively patchy clusters during the mating season (personal observation), which may drastically increase encounter rates between prospective mates, even when following trails in a random direction. Although field studies report males actively roaming the environment in the process of mate finding (Ghislandi et al. 2018), we lack studies on the mechanisms and cues used. While our males did not follow female trails directionally, we cannot fully exclude that our findings are driven by the artificial test environment. It is also possible that directional information is present in female silk trails, but males choose specific scenarios in which to follow a trail, for example, when sensing the trail was deposited by a female of high reproductive value, or males are unable to decode present directional information.
Nevertheless, given the higher proportions of males correctly following the trail when these remained unwashed, chemical attributes of trails may provide a potential means for conveying directionality information. Despite little being known about the chemical sensing and communication of P. mirabilis, for spiders in general, it is suggested to occur via contact-chemoreception through cuticular structures (i.e., sensilla) that are abundant on spider appendages, including pedipalps (Foelix 2010; Keil 2012). Hence, the observed probing both via legs and pedipalps in our high-speed video recordings may allow males detection of putative pheromones present in the silk or on the substrate (Bristowe and Locket 1926; Kaston 1936; Jackson 1983; Humbel et al. 2021). Gathering of information for initiating and possibly furthering directional trail-following was also suggested to play a role in two species of wolf spiders (Tietjen 1977), shown to repeatedly pull and probe the silk lines with their front legs and pedipalps. The exact mechanism for directionality in these species remains unknown. The author excluded the presence of a chemical gradient as the chemicals in these two species were stable over several weeks, reducing the detection of fine-tuned differences in concentration – that would be necessary for reliable directional information – due to evaporation (Tietjen 1977). Directionality in pheromone trails has only been described in very few cases, such as in ants, where it is known to be embedded by using trail geometry based on trail-bifurcations (Jackson et al. 2004). In contrast, spiders cannot use the same types of mechanisms since they lack bifurcating trails and an end-goal, such as the nest and food sources in ants. Alternatively, spiders may make use of a gradient of contact pheromones which inactivates rapidly after release (Baruffaldi et al. 2010). Yet, despite the importance of contact silk cues being acknowledged in this species (Beyer et al. 2018), the persistence of active chemicals in Pisaura mirabilis silk remains unknown.
The observed pulling of silk in the present work may also allow detecting variable tension around the silk attachment discs, which is necessary for inducing male directional trail-following (Tietjen 1977; Wolff et al. 2021). Position, angle, and morphology of a disc could result in differences in silk tension in the area surrounding the discs (Wolff et al. 2021) as, for example, the different mechanical robustness of the disc with respect to its upstream (direction toward silk layer) or downstream (direction toward the start of silk line) end could potentially be sensed by a trail-following individual (Wolff and Herberstein 2017). In order to assess differences in silk line tension, a male would be required to assess the tension around an immobilized portion of it by using its appendages. Explanations relying on chemical and structural (i.e., silk tension) assessment are not mutually exclusive; chemical signals may be coupled with mechanical orientation signals that are used in concert to find potential mates. While there is a potential that washing of the silk lines with ethanol could have also affected the structural and mechanical properties of the dragline that potentially play a role in trail directionality cues, we believe this to be unlikely. Any impact on the silk material would be global, i.e., any existing relative differences in upstream versus downstream draglines would have been maintained. Some spider silk types are known to transition into a rubbery state upon contact with water, thereby shrinking and increasing its diameter – a process called supercontraction (Liu et al. 2005; Stengel et al. 2020), and silk structure may also change after treatment with solvents (Beyer et al. 2021). However, our ultrastructural inspection of silk draglines (namely, major ampullate silk type) washed with solvents did not reveal visible structural changes in comparison to unwashed silk, such as an increase in silk diameter (see Online resource). The observed effect of trail washing on the prevalence of directional trail-following behavior is thus most likely explained by the removal of chemical cues only. Further research and especially chemo-analytical tests (e.g., chromatography) are needed to verify the removal of chemical information by washing with solvents.
Our study also revealed an effect of female phenotype on the correct trail-following of males, as males followed trails in the direction of females that were relatively larger in size – and avoided the direction of females that were relatively smaller – than themselves. Interestingly, in our first experiment, when exploring a potential role of female phenotypic traits, we found that the likelihood of male P. mirabilis to directionally follow females was conditional on female body mass, with 90% of the males following heaviest females (i.e., females of the quantile with highest absolute body mass) (Online resource). In our second experiment, when formally testing for the effects of variation in female phenotypic traits (body condition, mass, and size) obtained through experimental feeding manipulations on male directional trail-following, we failed to confirm these results. We believe results regarding the effect of female mass from experiment 1 warrant cautious interpretation, as, given the homogenous feeding conditions that females were given during rearing, natural variation in female body mass was overall low, leading to few high-mass females driving such a significant effect. On the contrary, the experimental procedure adopted in experiment 2, where female body mass and condition were experimentally manipulated through differential feeding regimes, coupled with a high sample size, is likely to be much more reliable.
The finding that male directional trail-following did not depend on female body mass, condition, or size remains, however, puzzling. We have hypothesized mass- and /or condition-related information to males stemming from varying amounts or composition of chemicals embedded in, or added to, the silk (Baruffaldi et al. 2010; Henneken et al. 2015, 2017; Weiss and Schneider 2022a), or from thicker silk threads (Vollrath 1999). Given the costs associated with nuptial gifts, we have also hypothesized selection favoring male discriminatory abilities of female cues. If information carried by female trails correlates with the female’s reproductive value, males should move toward females of higher body mass, condition, and size to safeguard their heavy investment in mating (Bonduriansky 2001). Pisaura mirabilis females of larger size and mass are known to be more fecund (Austad and Thornhill 1986; Stålhandske 2001; Pandulli-Alonso et al. 2022). In arthropods, male mate choice is often based on female fecundity (Bonduriansky 2001; Edward and Chapman 2011), a trait that is generally positively correlated with female body mass (or size) (Leather 2018), with mass itself being able to reflect recent food intake and subsequently the likelihood and timing of reproduction (e.g., egg laying) (Stoltz et al. 2010). In addition, in spiders, information on female mass is also used by males as a proxy for female satiation to assess the risk of cannibalism, with females of low mass (and thus low satiation) posing a greater risk than those of higher mass (Baruffaldi and Andrade 2015). Pisaura mirabilis females are likelier to cannibalize males when starved (Toft and Albo 2016). In one of our recent studies, we show that Pisaura mirabilis males discriminate between the silk of females varying in their body condition, with males exerting preference for the silk of females in higher compared to lower conditions (Beyer et al. 2023b). While the said study was conducted in a binary choice set up, with males simultaneously being exposed to the trails of a high- and low-condition female and did not include tests of directionality, it does show that there is variation in female silk and that males can and do in fact assess female phenotype exclusively from their trails.
Males were more likely to follow correctly relatively larger females, suggesting that – to some degree – males perceive relative differences in female phenotypes via their trails, and these are indicative of female movements. It is possible that male P. mirabilis can only probe the diameter of the silk line to infer female characteristics, and that larger (but not heavier) females produce larger silk threads. This would explain the effect of relative size, but not relative mass, on trail-following, despite evidence from orb-weavers indicating no effect of body size (but an effect of mass) on the diameter of radial silk threads (Vollrath and Kohler 1996). The perception of relative phenotypic differences would require individuals to self-assess their own size in relation to the size of another, an ability widespread in intra- and especially inter-sexual encounters (i.e., male-male conflicts) in arthropods (e.g., Taylor and Elwood 2003; Briffa 2008), including spiders (e.g., Wells 1988; Schaefer and Uhl 2003; Taylor and Jackson 2003). Such relative size-dependent self-assessment is already hypothesized in female P. mirabilis that allows longer copulations to relatively smaller males (Prokop 2006). Relatively smaller males also gain relatively higher paternity shares (Matzke et al. 2022). Their advantage may be explained by higher agility (Blanckenhorn 2000) and potentially higher mechanical compatibility in entering the mating position (Dufour 1844; Masly 2012; Xia et al. 2023). If male P. mirabilis use a putative self-assessment ability in relation to the female’s size, larger females may be preferred and followed. Perception of potential incompatibility may drive male avoidance of trails deposited by smaller females whose genitalia (e.g., epigynal opening) are either difficult to reach or enter with a comparatively large males’ pedipalps due to females being comparatively too small, likely consequently resulting in the reduced success of copulation and/or insemination (Schick 1965). In contrast, the perception of compatibility might increase the male likelihood to follow trails of females that are relatively larger, e.g., due to eased access to the female’s genitalia. We can only speculate whether mechanisms involve the interaction between relatively smaller male sensory appendages in smaller males and enhanced chemical and/or structural trail properties of larger females signaling directionality. We note that despite having a large sample size, the body sizes in this study represent natural variation, with a median (and mean) relative size difference of male–female testing pairs of approximately 1, as males and females in this species are of similar size. Further research might be necessary in which body size in male and female testing pairs is manipulated, especially since strength of male choice regarding female quality (e.g., large size) is known to be higher for medium- and high-quality males (Pollo et al. 2022).
To conclude, our tests on male trail-following provide no overwhelming evidence of general directionality in male movements during trail-following. Male spiders use silk trails as guides for movements, and the gathered chemical and tactile cues might encode information on female quality. Directionality appears to be present only under certain conditions, with chemical attributes of trails having a potentially key role in providing males with size-dependent information on female movements. The natural history of this species (relatively clustered populations with high densities) may weaken mechanisms for embedding trails with directionality in females or detecting them in males, as these would require costly sensory machinery in the face of increased predation and parasitism risks. These results contribute to the lack of empirical studies on directionality and are key for advancing our understanding of mechanisms involved in animal communication and mate searching in free-living and solitary species of arthropods. Decoding of directional information remains an exciting venue for research. Finally, we emphasize the importance of accounting both for male and female interacting phenotypes to understand their influence on reproductive behaviors. Our findings that male trail-following is further influenced by indicators of relative size differences between the sexes point to a scenario of relative assessment potentially driven by male mate choice in a system characterized by high costs of mating via male nuptial gifts.
Data availability
All data generated or analyzed are included in the Supplementary information.
References
Albo MJ, Toft S, Bilde T (2011) Condition dependence of male nuptial gift construction in the spider Pisaura mirabilis (Pisauridae). J Ethol 29(3):473–479. https://doi.org/10.1007/s10164-011-0281-1
Anderson JT, Morse DH (2001) Pick-up lines: cues used by male crab spiders to find reproductive females. Behav Ecol 12(3):360–366. https://doi.org/10.1093/beheco/12.3.360
Apstein C (1899) Bau und Funktion der Spinndrüsen der Araneida. Arch Für Naturgeschichte 55:29–74
Austad SN, Thornhill R (1986) Female reproductive variation in a nuptial-feeding spider, Pisaura mirabilis. BullBr.arachnolSoc 7(2):48–52
Barth FG (1993) Sensory guidance in spider pre-copulatory behaviour. Comp Biochem Physiol Part A Physiol 104(4):717–733. https://doi.org/10.1016/0300-9629(93)90148-W
Baruffaldi L, Andrade MCB (2015) Contact pheromones mediate male preference in black widow spiders: avoidance of hungry sexual cannibals? Anim Behav 102:25–32. https://doi.org/10.1016/j.anbehav.2015.01.007
Baruffaldi L, Costa FG (2010) Changes in male sexual responses from silk cues of females at different reproductive states in the wolf spider Schizocosa malitiosa. J Ethol 28(1):75–85. https://doi.org/10.1007/s10164-009-0158-8
Baruffaldi L, Costa FG, Rodríguez A, González A (2010) Chemical communication in Schizocosa malitiosa: evidence of a female contact sex pheromone and persistence in the field. J Chem Ecol 36(7):759–767. https://doi.org/10.1007/s10886-010-9819-x
Bell WJ (1990) Searching behavior patterns in insects. Annu Rev Entomol 35(1):447–467. https://doi.org/10.1146/annurev.en.35.010190.002311
Bell RD, Roberts JA (2016) Trail-following behavior by males of the wolf spider, Schizocosa ocreata (Hentz). J Ethol 35(1):29–36. https://doi.org/10.1007/s10164-016-0486-4
Beyer M, Czaczkes TJ, Tuni C (2018) Does silk mediate chemical communication between the sexes in a nuptial feeding spider? Behav Ecol Sociobiol 72(3):49–56. https://doi.org/10.1007/s00265-018-2454-1
Beyer M, Mangliers J, Tuni C (2021) Silk-borne chemicals of spider nuptial gifts elicit female gift acceptance. Biol Lett. https://doi.org/10.1098/rsbl.2021.0386
Beyer M, Uludag KÖ, Lailler M, Eberhard MJB, Wolff JO, Czaczkes TJ, Tuni C (2023a) Data from: do female spiders embed silk trails with information on their movement direction and phenotype, and do males follow trails accordingly? Figshare Behav Ecol Sociobiol. https://doi.org/10.6084/m9.figshare.19174058.v2
Beyer M, Uludağ KO, Tuni C (2023b) Female state and condition-dependent chemical signaling revealed by male choice of silk trails. Behav Ecol. https://doi.org/10.1093/beheco/arad068
Bilde T, Tuni C, Elsayed R, Pekar S, Toft S (2007) Nuptial gifts of male spiders: sensory exploitation of the female’s maternal care instinct or foraging motivation? Anim Behav 73(2):267–273. https://doi.org/10.1016/j.anbehav.2006.05.014
Blanckenhorn WU (2000) The evolution of body size: what keeps organisms small? Q Rev Biol 75(4):385–407
Bonduriansky R (2001) The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev Camb Philos Soc 76(3):305–339. https://doi.org/10.1017/S1464793101005693
Bordereau C, Pasteels JM (2010) Pheromones and chemical ecology of dispersal and foraging in termites. Biology of Termites: a Modern Synthesis. Springer, Netherlands, Dordrecht, pp 279–320
Bradbury JW, Vehrencamp SL (2009) Principles of animal communication. 2nd ed. Massachussets: Sinauer Associates, Inc. http://sites.sinauer.com/animalcommunication2e/index.html.
Briffa M (2008) Decisions during fights in the house cricket, Acheta domesticus: mutual or self assessment of energy, weapons and size? Anim Behav 75(3):1053–1062. https://doi.org/10.1016/j.anbehav.2007.08.016
Bristowe WS, Locket GH (1926) The courtship of British lycosid spiders, and its probable significance. Proc Zool Soc London 96(1):317–347
Butt BA, Hathaway DO (1966) Female sex pheromone as attractant for male codling moths. J Econ Entomol 59(2):476–477. https://doi.org/10.1093/jee/59.2.476
Chinta S, Goller S, Lux J, Funke S, Uhl G, Schulz S (2010) The sex pheromone of the wasp spider Argiope bruennichi. Angew Chemie (march). https://doi.org/10.1002/anie.200906311
Cook SB, Cook CB (1975) Directionality in the trail-following response of the pulmonate limpet Siphonaria alternata. Mar Behav Physiol 3(3):147–155. https://doi.org/10.1080/10236247509378506
Cooper WE, Vitt LJ (1986) Tracking of female conspecific odor trails by male broad-headed skinks (Eumeces laticeps). Ethology 71(3):242–248. https://doi.org/10.1111/j.1439-0310.1986.tb00587.x
Cory AL, Schneider JM (2016) Old maids have more appeal: effects of age and pheromone source on mate attraction in an orb-web spider. PeerJ 2016(4) https://doi.org/10.7717/peerj.1877
Craig CL, Riekel C, Herberstein ME, Weber RS, Kaplan D, Pierce NE (2000) Evidence for diet effects on the composition of silk proteins produced by spiders. Mol Biol Evol 17(12):1904–1913. https://doi.org/10.1093/oxfordjournals.molbev.a026292
Czaczkes TJ, Grüter C, Ratnieks FLW (2015) Trail pheromones: an integrative view of their role in social insect colony organization. Annu Rev Entomol 60:581–599. https://doi.org/10.1146/annurev-ento-010814-020627
Danielson-Francois A, Fetterer CA, Smallwood PD (2002) Body condition and mate choice in Tetragnatha elongata ( Araneae, Tetragnathidae ). J Arachnol 30(1):20–30
Dijkstra H (1976) Searching behaviour and tactochemical orientation in males of the wolfspider Pardosa amentata (Cl.) (Araneae, Lycosidae). Entomology 235–244
Dufour L (1844) Anatomie générale des Dipteres. Ann Des Sci Nat 1:244–264
Eberhard MJB, Möller TA, Uhl G (2021) Dragline silk reveals female developmental stage and mediates male vibratory courtship in the nuptial gift-giving spider Pisaura mirabilis. Ethology 127(3):267–277. https://doi.org/10.1111/eth.13124
Edward DA, Chapman T (2011) The evolution and significance of male mate choice. Trends Ecol Evol 26(12):647–654. https://doi.org/10.1016/j.tree.2011.07.012
Edwards M, Davies MS (2002) Functional and ecological aspects of the mucus trails of the intertidal prosobranch gastropod Littorina littorea. Mar Ecol Prog Ser 239(Calow 1974):129–137. https://doi.org/10.3354/meps239129
Fei M, Gols R, Harvey JA (2023) The biology and ecology of parasitoid wasps of predatory arthropods. Annu Rev Entomol 68:109–128. https://doi.org/10.1146/annurev-ento-120120-111607
Fischer A (2019) Chemical communication in spiders – a methodological review. J Arachnol 47(1):1. https://doi.org/10.1636/0161-8202-47.1.1
Fischer A, Schulz S, Ayasse M, Uhl G (2021) Pheromone communication among sexes of the garden cross spider Araneus diadematus. Sci Nat 108(5):1–11. https://doi.org/10.1007/s00114-021-01747-9
Foelix R (2010) Biology of spiders. Oxford University Press
Ford NB, Low JR (1984) Sex pheromone source location by garter snakes: a mechanism for detection of direction in novolatile trails. J Chem Ecol 10(8):1193–1199. https://doi.org/10.1007/BF00988548
Forrest TG (1982) Acoustic communication and baffling behaviors of crickets. Florida Entomol 65(1):33–44. https://doi.org/10.2307/3494144
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Br Ecol Soc 7(11):1325–1330
Gehlbach FR, Watkins JF, Kroll JC (1971) Pheromone trail-following studies of typhlopid, leptotyphlopid, and colubrid snakes. Behaviour 40(3–4):282–294. https://doi.org/10.1163/156853971X00429
Gerbaulet M, Möllerke A, Weiss K, Chinta S, Schneider JM, Schulz S (2022) Identification of cuticular and web lipids of the spider Argiope bruennichi. J Chem Ecol 48(3):244–262. https://doi.org/10.1007/s10886-021-01338-y
Ghislandi PG, Beyer M, Velado P, Tuni C (2017) Silk wrapping of nuptial gifts aids cheating behaviour in male spiders. Behav Ecol 28(3):744–749. https://doi.org/10.1093/beheco/arx028
Ghislandi PG, Pekár S, Matzke M, Schulte-Döinghaus S, Bilde T, Tuni C (2018) Resource availability, mating opportunity and sexual selection intensity influence the expression of male alternative reproductive tactics. J Evol Biol. 31(7)https://doi.org/10.1111/jeb.13284.
Goodrich B, Gabry J, Ali I, Brilleman S (2020) rstanarm: Bayesian applied regression modeling via Stan. R package version 2(1)
Harmsen BJ, Foster RJ, Silver S, Ostro L, Doncaster CP (2010) Differential use of trails by forest mammals and the implications for camera-trap studies: a case study from Belize. Biotropica 42(1):126–133. https://doi.org/10.1111/j.1744-7429.2009.00544.x
Hegdekar BM, Dondale CD (1969) A contact sex pheromone and some response parameters in lycosid spiders. Can J Zool 47(1):1–4. https://doi.org/10.1139/z69-001
Henneken J, Jones TM, Goodger JQD, Dias DA, Walter A, Elgar MA (2015) Diet influences female signal reliability for male mate choice. Anim Behav 108:215–221. https://doi.org/10.1016/j.anbehav.2015.07.023
Henneken J, Goodger JQD, Jones TM, Elgar MA (2017) Diet-mediated pheromones and signature mixtures can enforce signal reliability. Front Ecol Evol. 4(JAN) https://doi.org/10.3389/fevo.2016.00145.
Hinde RA (1971) Bird vocalizations: their relations to current problems in biology and psychology: essays presented to WH Thorpe. Cambridge University Press, New York
Howard JJ (2001) Costs of trail construction and maintenance in the leaf-cutting ant Atta columbica. Behav Ecol Sociobiol 49(5):348–356. https://doi.org/10.1007/s002650000314
Humbel EA, Kimball RT, Taylor LA (2021) Males respond to substrate-borne, not airborne, female chemical cues in the jumping spider, Habronattus pyrrithrix (Araneae: Salticidae). J Arachnol 49(2):262–267. https://doi.org/10.1636/JoA-S-20-055
Jackson RR (1983) The biology of Mopsus mormon, a jumping spider (Araneae: Salticidae) from Queensland: intraspecific interactions. Aust J Zool 31(1):39–53. https://doi.org/10.1071/ZO9830039
Jackson RR (1987) Comparative study of releaser pheromones associated with the silk of jumping spiders (Araneae, Salticidae). New Zeal J Zool 14(1):1–10. https://doi.org/10.1080/03014223.1987.10422676
Jackson DE, Holcombe M, Ratnieks FLW (2004) Trail geometry gives polarity to ant foraging networks. Nature 432(7019):907–909. https://doi.org/10.1038/nature03105
Jakob EM, Marshall SD, Uetz GW (1996) Estimating fitness: a comparison of body condition indices. Oikos 77(1):61–67. https://doi.org/10.2307/3545585
Jamon M (1994) An analysis of trail-following behaviour in the wood mouse. Anim Behav 47(5):1127–1134. https://doi.org/10.1006/anbe.1994.1151Get
Jarau S (2009) Chemical communication during food exploitation in stingless bees. Food Exploit by Soc Insects Ecol Behav Theor Approaches 1990:223–250. https://doi.org/10.1201/9781420075618
Jeanne RL (1981) Chemical communication during swarm emigration in the social wasp Polybia sericea (Olivier). Anim Behav 29(1):102–113. https://doi.org/10.1016/S0003-3472(81)80157-1
Johnson JC, Miles LS, Trubl PJ, Hagenmaier A (2014) Maternal effects on egg investment and offspring performance inblack widow spiders. Anim Behav 91:67–73. https://doi.org/10.1016/j.anbehav.2014.02.031
Karlson P, Lüscher M (1959) “Pheromones”: a new term for a class of biologically active substances. Nature 183:55–56
Kaston BJ (1936) The senses involved in the courtship of some vagabond spiders. Entomologica Americana 16:97–167
Keil TA (2012) Sensory cilia in arthropods. Arthropod Struct Dev 41(6):515–534. https://doi.org/10.1016/j.asd.2012.07.001
Lang A (1996) Silk investment in gifts by males of the nuptial feeding spider Pisaura mirabilis (Araneae: Pisauridae). Behavior 133(9):697–716
Leather SR (2018) Factors affecting fecundity, fertility, oviposition and larviposition in insects. Insect Reproduction. CRC Press, Boca-Raton, Florida, pp 143–174
Liu Y, Shao Z, Vollrath F (2005) Relationships between supercontraction and mechanical properties of spider silk. Nat Mater 4(12):901–905. https://doi.org/10.1038/nmat1534
Magris M, Tuni C (2019) Enough for all: no mating effort adjustment to varying mate availability in a gift-giving spider. Behav Ecol 30(5):1461–1468. https://doi.org/10.1093/beheco/arz102
Masly JP (2012) 170 Years of “lock-and-key”: genital morphology and reproductive isolation. Int J Evol Biol 2012:1–10. https://doi.org/10.1155/2012/247352
Matzke M, Toft S, Bechsgaard J, Pold Vilstrup A, Uhl G, Künzel S, Tuni C, Bilde T (2022) Sperm competition intensity affects sperm precedence patterns in a polyandrous gift-giving spider. Mol Ecol 31(8):2435–2452. https://doi.org/10.1111/mec.16405
Müller CHG, Ganske AS, Uhl G (2020) Ultrastructure of chemosensory tarsal tip-pore sensilla of Argiope spp. Audouin, 1826 (Chelicerata: Araneae: Araneidae). J Morphol. 281(12):1634–1659. https://doi.org/10.1002/jmor.21276
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82(4):591–605. https://doi.org/10.1111/j.1469-185X.2007.00027.x
Ng TPT, Saltin SH, Davies MS, Johannesson K, Stafford R, Williams GA (2013) Snails and their trails: the multiple functions of trail-following in gastropods. Biol Rev 88(3):683–700. https://doi.org/10.1111/brv.12023
Nieh JC, Contrera FAL, Yoon RR, Barreto LS, Imperatriz-Fonseca VL (2004) Polarized short odor-trail recruitment communication by a stingless bee, Trigona Spinipes. Behav Ecol Sociobiol 56(5):435–448. https://doi.org/10.1007/s00265-004-0804-7
Nitzsche ROM (1988) ‘Brautgeschenk’ und Umspinnen der Beute bei Pisaura mirabilis, Dolomedes fimbriatus und Thaumasia uncata (Arachnida, Araneida, Pisauridae). Verhandlungen Des Naturwissenschaftlichen Vereins Hambg 30:353–393
O’Donnell RP, Ford NB, Shine R, Mason RT (2004) Male red-sided garter snakes, Thamnophis sirtalis parietalis, determine female mating status from pheromone trails. Anim Behav 68(4):677–683. https://doi.org/10.1016/j.anbehav.2003.09.020
Pandulli-Alonso I, Tomasco IH, Albo MJ (2022) The handsome liar: male spiders offering worthless gifts can benefit increasing mating duration. Ethology 128(3):215–222. https://doi.org/10.1111/eth.13258
Pollo P, Nakagawa S, Kasumovic MM (2022) The better, the choosier: a meta-analysis on interindividual variation of male mate choice. Ecol Lett 25(5):1305–1322. https://doi.org/10.1111/ele.13981
Prokop P (2006) Insemination does not affect female mate choice in a nuptial feeding spider. Ital J Zool 73(3):197–201. https://doi.org/10.1080/11250000600727741
Prokop P, Okrouhlík J (2021) Metabolic cost of holding nuptial food gifts for male spiders. Ecol Entomol 46(3):684–690
R Core Team (2021) R: a language and environment for statistical computing. https://www.r-project.org/
Rosengren R, Fortelius W (1987) Trail communication and directional recruitment to food in red wood ants (Formica). Ann Zool Fennici 24(2):137–146
Ross K, Smith RL (1979) Aspects of the courtship behavior of the black widow spider, Latrodectus hesperus (Araneae : Theridiidae), with evidence for the existence of a contact sex pheromone. Am Arachnol Soc 7:69–77
Rovner JS, Barth FG (1981) Vibratory communication through living plants by a tropical wandering spider. Science (80-) 214(4519):464–466. https://doi.org/10.1126/science.214.4519.464
Rutowski R, Demlong M, McCoy L (2001) Visual mate detection in a territorial male butterfly (Asterocampa leilia): effects of distance and perch location. Behavior 138(1):31–43. https://doi.org/10.1163/156853901750077772
Schaefer D, Uhl G (2003) Male competition over access to females in a spider with last-male sperm precedence. Ethology 109(5):385–400. https://doi.org/10.1046/j.1439-0310.2003.00881.x
Schick RX (1965) The crab spiders of California. Bull Am Mus Nat Hist 129:1–180
Schulz S (2013) Spider pheromones - a structural perspective. J Chem Ecol 39(1):1–14. https://doi.org/10.1007/s10886-012-0231-6
Scott CE, McCann S, Andrade MCB (2019) Male black widows parasitize mate-searching effort of rivals to find females faster. Proc R Soc B Biol Sci 286(1908) https://doi.org/10.1098/rspb.2019.1470.
Shao Z, Vollrath F (1999) The effect of solvents on the contraction and mechanical properties of spider silk. Polymer (guildf) 40(7):1799–1806. https://doi.org/10.1016/S0032-3861(98)00266-3
Stålhandske P (2001) Nuptial gift in the spider Pisaura mirabilis maintained by sexual selection. Behav Ecol 12(6):691–697. https://doi.org/10.1093/beheco/12.6.691
Stengel D, Addison JB, Onofrei D, Huynh NU, Youssef G, Holland GP (2020) Hydration-induced beta-sheet crosslinking of alpha-helical-rich spider prey-wrapping silk. Adv Funct Mater.(2007161)https://doi.org/10.1002/adfm.202007161
Stevens M (2013) Sensory ecology, information, and decision-making. Sensory ecology, information, and decision-making. OUP, Oxford, pp 2–18
Stoltz JA, Ramez H, Andrade MCB (2010) Longevity cost of remaining unmated under dietary restriction. Funct Ecol 1270–1280 https://doi.org/10.1111/j.1365-2435.2010.01729.x
Suter RB, Renkes G (1982) Linyphid spider courtship: releaser and attractant functions of a contact sex pheromone. Anim Behav 30(3):714–718. https://doi.org/10.1016/S0003-3472(82)80142-5
Suter RB, Shane C, Hirscheimer A (1987) Communication by cuticular pheromones in a linyphiid spider. J Arachnol 15(2):157–162. https://doi.org/10.2307/3705724
Taylor PW (1998) Dragline-mediated mate-searching in Trite planiceps (Araneae, Salticidae ). J Arachnol 26(3):330–334
Taylor PW, Elwood RW (2003) The mismeasure of animal contests. Anim Behav 65(6):1195–1202. https://doi.org/10.1006/anbe.2003.2169
Taylor PW, Jackson RR (2003) Interacting effects of size and prior injury in jumping spider conflicts. Anim Behav 65(4):787–794. https://doi.org/10.1006/anbe.2003.2104
Tietjen WJ (1977) Dragline-following by male lycosid spiders. Psyche A J Entomol 84(2):165–178. https://doi.org/10.1155/1977/29581
Tietjen WJ, Rovner JS (1980) Trail-following behaviour in two species of wolf spiders: sensory and etho-ecological concomitants. Anim Behav 28(3):735–741. https://doi.org/10.1016/S0003-3472(80)80133-3
Todt D, Naguib M (2000) Vocal interactions in birds: the use of song as a model in communication. In: Adv Study Behav 29(Academic Press):247–296
Toft S, Albo MJ (2015) Optimal numbers of matings: the conditional balance between benefits and costs of mating for females of a nuptial gift-giving spider. J Evol Biol 28(2):457–467
Toft S, Albo MJ (2016) The shield effect: nuptial gifts protect males against pre-copulatory sexual cannibalism. Biol Lett 12(5):20151082. https://doi.org/10.1098/rsbl.2015.1082
Tso IM, Wu HC, Hwang IR (2005) Giant wood spider Nephila pilipes alters silk protein in response to prey variation. J Exp Biol 208(6):1053–1061. https://doi.org/10.1242/jeb.01437
Tuni C, Albo MJ, Bilde T (2013) Polyandrous females acquire indirect benefits in a nuptial feeding species. J Evol Biol 26(6):1307–1316. https://doi.org/10.1111/jeb.12137
van Baarlen P, Topping CJ, Sunderland KD (1996) Host location by Gelis festinans, an eggsac parasitoid of the linyphiid spider Erigone atra. Entomol Exp Appl 81(2):155–163. https://doi.org/10.1111/j.1570-7458.1996.tb02027.x
van der Kraan C, Richter CJJ (1970) Silk production in adult males of the wolf spider Pardosa amentata (Cl.) (Araneae, Lycosidae). Netherlands J Zool. 20(3):392–400
Virant-Doberlet M, Cokl A (2004) Vibrational communication in insects. Neotrop Entomol 33(2):121–134. https://doi.org/10.1590/S1519-566X2004000200001
Vollrath F (1999) Biology of spider silk. Int J Biol Macromol 24(2–3):81–88. https://doi.org/10.1016/S0141-8130(98)00076-2
Vollrath F, Kohler T (1996) Mechanics of silk produced by loaded spiders. Proc R Soc B Biol Sci 263(1369):387–391. https://doi.org/10.1098/rspb.1996.0059
Vong A, Ansart A, Dahirel M (2019) Dispersers are more likely to follow mucus trails in the land snail Cornu aspersum. Sci Nat 106(7–8)https://doi.org/10.1007/s00114-019-1642-9
Watson PJ (1986) Transmission of a female sex pheromone thwarted by males in the spider Linyphia litiqiosa (Inypblidae). Science (80-) 233(4760):219–221. https://doi.org/10.1126/science.3726530
Weatherston J, Percy JE (1977) Sex pheromones of moths. Endeavour 1(2):83–87. https://doi.org/10.1016/0160-9327(77)90111-9
Weiss K, Schneider JM (2022a) Strategic pheromone signalling by mate searching females of the sexually cannibalistic spider Argiope bruennichi. R Soc Open Sci 9(1)https://doi.org/10.1098/rsos.211806
Weiss K, Schneider JM (2022b) Female sex pheromone emission is affected by body condition, but not immune system function, in the orb-web spider Argiope bruennichi. Ethology 128(6):471–481. https://doi.org/10.1111/eth.13280
Wells MS (1988) Effects of body size and resource value on fighting behaviour in a jumping spider. Anim Behav 36(2):321–326. https://doi.org/10.1016/S0003-3472(88)80001-0
Wignall AE, Taylor PW (2009) Responses of an araneophagic assassin bug, Stenolemus bituberus, to spider draglines. Ecol Entomol 34(3):415–420. https://doi.org/10.1111/j.1365-2311.2009.01088.x
Wilson EO (1962) Chemical communication among workers of the fire ant Solenopsis saevissima. Anim Behav. 10(1–2) https://doi.org/10.1016/0003-3472(62)90141-0
Witt PN (1975) The web as a means of communication. Biosci Commun 1:7–23
Wolff JO, Michalik P, Ravelo AM, Herberstein ME, Ramírez MJ (2021) Evolution of silk anchor structure as the joint effect of spinning behavior and spinneret morphology. Integr Comp Biol 61(4):1411–1431. https://doi.org/10.1093/icb/icab003
Wolff JO, Herberstein ME (2017) Three-dimensional printing spiders: back-and-forth glue application yields silk anchorages with high pull-off resistance under varying loading situations. J R Soc Interface 14(127) https://doi.org/10.1098/rsif.2016.0783
Xia T, Nishimura T, Nagata N, Kubota K, Sota T, Takami Y (2023) Reproductive isolation via divergent genital morphology due to cascade reinforcement in Ohomopterus ground beetles. J Evol Biol 36(1):169–182. https://doi.org/10.1111/jeb.14116
Yoshida H, Suzuki Y (1981) Silk as a cue for mate location in the jumping spider, Carrhotus xanthogramma (Latreille) (Araneae: Salticidae). Appl Entomol Zool 16(3):315–317. https://doi.org/10.1303/aez.16.315
Acknowledgements
We thank Julia Mangliers for the assistance in collecting spiders and helping run trials for experiment 1. The study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) (470442873) to Cristina Tuni. Michelle Beyer’s visit to Greifswald was funded by a travel grant of the Ethologische Gesellschaft e.V. Tomer J. Czaczkes was funded by a Heisenberg fellowship (462101190). Jonas Wolff was supported by the DFG (Grant 451087507).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
CT, MB, and TC conceived and designed the study; KÖU collected data for experiment 1; ML and MB collected data for experiment 2; MB collected data for experiment 3; MJE and JOW provided logistics and assisted with data collection for experiment 3; MB drafted the manuscript and analyzed data; CT and TC contributed to writing and editing. All authors have read, provided comments, and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by N. Wedell
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beyer, M., Uludag, K.Ö., Lailler, M. et al. Testing presence of directionality information in female spider silk trails through male trail-following behavior. Behav Ecol Sociobiol 77, 139 (2023). https://doi.org/10.1007/s00265-023-03386-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03386-8