Abstract
Obligate brood parasites lay their eggs in nests of other species, with host parents bearing the cost of raising their offspring. These costs imposed on hosts select for the evolution of host defenses against parasitism at all stages of the reproductive cycle. The most effective defense is egg rejection at early stages of the breeding cycle, with later-stage defenses (nestling and fledgling discrimination) being less common. In this study, we tested whether the hoopoe (Upupa epops), a potential host of the great spotted cuckoo (Clamator glandarius) without egg rejection ability, presents defenses after the egg stage. We experimentally parasitized hoopoe nests with great spotted cuckoo nestlings creating mixed broods (with hoopoe and cuckoo nestlings) and broods with only cuckoo nestlings and measured parental feeding behavior and survival of nestlings and fledglings of both species. Cuckoo fledglings were fed fewer often than hoopoe fledglings in mixed broods, and adults approached more often to feed hoopoe fledglings than cuckoo fledglings. Consequently, the survival of cuckoo fledglings in both mixed and only-cuckoo-broods, was significantly lower than that of hoopoe fledglings. These results suggest that hoopoes would discriminate great spotted cuckoo fledglings, with or without direct comparison with their own fledglings. However, the survival of some cuckoos suggests that hoopoes have not reached highly efficient defenses so, other life history traits hindering parasitism by cuckoos may explain low parasitism rates and low levels of defenses in this species.
Significance statement
Brood parasites lay their eggs in nests of other species, tricking hosts into raising their parasitic offspring. However, hosts may fight back impeding successful parasitism by developing defences at any of the stages of their breeding cycle. We investigated why the hoopoe is not parasitized by the great spotted cuckoo despite this potential host apparently does not show such anti-parasitic defenses. We found that hoopoes have evolved the less common host defense: discrimination of parasite fledglings, even in the absence of their own fledgling for comparison. Our study supports the idea that discrimination during the later stages of the nesting cycle (i.e. nestling and fledgling periods) may be more common that previously assumed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obligate brood parasite females lay their eggs in the nests of other species (a type of parental-care parasitism: Roldán and Soler 2011). This means that brood parasites evade all parental care, fooling hosts into raising their parasitic offspring and thus diverting resources away from their own nestlings (Soler 2017a). Brood parasitic nestlings usually outcompete host nestlings because they hatch earlier, are larger and beg at a higher intensity than host nestlings (Soler 2017b). Therefore, brood parasitism imposes important fitness costs on hosts, reducing their reproductive success to nearly zero in many cases (Moskát et al. 2017). These severe costs imposed by brood parasites on hosts select for the evolution of host defenses, which in turn drives selection for the brood parasite to evolve counter-defenses, triggering improved host defenses, further parasitic adaptations and so on (Davies 2000; Soler and Soler 2000; Stokke et al. 2005; Feeney et al. 2014; Soler 2014). These reciprocal evolutionary changes, so-called coevolutionary arms race, have proven to be excellent systems for understanding coevolution, in which some of the clearest experimental demonstrations of a coevolutionary process have been reported (e.g. Brooke and Davies 1988; Soler and Møller 1990).
Despite egg-rejection being the most common and effective host defense against brood parasites, nowadays it is widely accepted that adaptations and counter-adaptations may occur at any stage of the breeding cycle; moreover, defenses at an early stage can influence the evolution of subsequent defenses and counter-defenses at later stages (Davies 2011; Feeney et al. 2014; Soler 2014, 2017a). An efficient line of defense at an earlier stage of the breeding cycle would be more beneficial because costs of brood parasitism accumulate as the breeding cycle advances (Britton et al. 2007; Davies 2011; Feeney et al. 2014; Soler 2014, 2017a). Such early lines of defense would prevent the evolution of later-stage defenses (nestling and fledgling discrimination) which may explain why the latter is less common than the former (frontline defenses and egg rejection). Nevertheless, when effective defenses have not evolved at earlier stages, a host defense at a later stage of the breeding cycle would be more likely to evolve (Britton et al. 2007; Feeney et al. 2012, 2014; Soler 2017a).
The great spotted cuckoo (Clamator glandarius) is a non-evictor specialist brood parasite which, in the Palearctic, lay most of their eggs in the nests of its main host: the common magpie (Pica pica), with carrion crow (Corvus corone) being its secondary host. Other corvid and non-corvid species are also parasitized in some populations (Soler 1990; Johnsgard 1997; Erritzøe et al. 2012). The coevolutionary relationships between the great spotted cuckoo and its hosts are well studied (Soler and Soler 2000; Roldán et al. 2013a; Bolopo et al. 2015). This brood parasite chooses brood-reducing host species that selectively feed larger nestlings (Soler 2001, 2002, 2017b). The appearance of great spotted cuckoo eggs does not mimic that of the host eggs (Soler et al. 2003), and there is no evidence for begging-call mimicry of hosts by great spotted cuckoo nestlings either (Roldán et al. 2013b). The average body mass of cuckoo females is 152 g and that of males is 157 (Macías-Sánchez et al. 2013). The magpie is slightly larger than the great spotted cuckoo while the carrion crow is more than twice the weight of the brood parasite (Soler et al. 2002). When parasitizing the magpie, the breeding success of the parasite is very high and that of the host very low (on average, only 0.7 magpie nestlings fledge per parasitized nest compared to 3.6 magpie nestlings that fledge in unparasitized nest (Soler et al. 2002)). Conversely, the cost of brood parasitism is lower in the carrion crows (on average 1.6 carrion crow nestlings fledge per parasitized nest compared to 3.1 in unparasitized nests (Soler et al. 2002)); the larger size of host nestlings makes cuckoo nestlings less efficient in obtaining food from their foster parents than their larger host nest mates (Bolopo et al. 2015).
The hoopoe (Upupa epops) is a hole-nesting bird with biparental care that presents a marked asynchronous hatching pattern (Cramp 1998; Martín-Vivaldi et al. 1999; Hoffmann et al. 2015; Ryser et al. 2016; Hildebrandt and Schaub 2018). It is a medium-sized bird, with a body mass ranging from 76.6 g in males to 73.1 g in females (Hildebrandt and Schaub 2018). The hoopoe could be a suitable host species for the great spotted cuckoo because the hoopoe follows a brood-reduction strategy, as hosts of the great spotted cuckoo do (Soler 2017b), and feeds its nestlings and fledglings with insects, an appropriate diet for this brood parasite. Cavity-nesting species have been traditionally considered unsuitable common cuckoo (Cuculus canorus) hosts (Davies and Brooke 1989b), because the small size of the nest entrance prevents the female cuckoo from laying her eggs effectively (Davies and Brooke 1989a; Moreras et al. 2021) or the young cuckoos leaving the nest at fledging time (Löhrl 1979). However, the cavities used by hoopoes many times have a larger entrance that may allow the entry of great spotted cuckoo females. In fact, the great spotted cuckoo can use other hole-nesters of medium size as hosts like jackdaws (Corvus monedula) and choughs (Pyrrhocorax pyrrhocorax) in Spain (Soler 1990) or Cape starlings (Lamprotornis nitens) and African pie starlings (Spreo bicolor) in South Africa (Johnsgard 1997; Erritzøe et al. 2012). However, to the best of our knowledge, there is only one record of possible hoopoe parasitism by the great-spotted cuckoo in Spain. In that record, hoopoe adults were observed feeding a great-spotted cuckoo fledgling in the wild (Amor-García et al. 2020). Even though the authors did not provide direct evidence that hoopoes reared this cuckoo nestling in their own nest, this observation points to the possibility of hoopoes being a potential host for this brood parasite.
Potential host defenses against brood parasitism in the hoopoe have not been thoroughly studied. Although appropriate egg-recognition experiments have not been performed in the hoopoe, experiments with other purposes that needed the introduction of foreign eggs in natural nests (Díaz-Lora et al. 2021) or introduced collared dove (Streptopelia decaocto) eggs and plaster model eggs in captivity nests (MM-V et al., unpublished data), never resulted in egg rejection. Thus, we can assume that egg rejection has not evolved in this potential great spotted cuckoo host species. In the Guadix area (a population located in southern Spain), the great spotted cuckoo reaches a high breeding density with parasitism of four different host species (Soler 1990). Moreover, hundreds of hoopoe nests have been monitored, both in natural cavities and nest-boxes (Martín-Vivaldi et al. 1999, 2006, 2009; Ruiz-Rodríguez et al. 2013; Díaz-Lora et al. 2019, 2021). In spite of these favorable traits of the hoopoe as a host and the intense monitoring of both reproductive hoopoes and great spotted cuckoos, no trace of great spotted cuckoo parasitism in the hoopoe was found in our study area.
This observation suggests that the hoopoe would have already evolved efficient defenses against brood parasitism during the nestling or during the fledging periods, which could prevent successful parasitism by the great spotted cuckoo. This possibility is quite plausible given that nowadays it is broadly accepted that adaptations and counteradaptations in brood parasites and their hosts can evolve at all phases of the nesting cycle (Soler 2014). This is the main objective of our study: to determine whether the hoopoe, a potential host of the great spotted cuckoo without egg rejection ability, presents defenses after the egg stage. We explored this possibility by proposing two mutually exclusive hypotheses. First, the hoopoe may have evolved nestling discrimination, a potential host defense less common than egg discrimination, which is predicted to occur more frequently when an efficient adaptation at an earlier stage has not evolved (the blocking model: Britton et al. 2007). In fact, most of the hosts that discriminate parasite nestlings do not discriminate parasite eggs (Grim 2017). As the hoopoe does not have the ability to recognize foreign eggs, our first hypothesis is that the hoopoe has evolved nestling discrimination. Our second hypothesis is that fledgling discrimination could be the host defense responsible for preventing success of great spotted cuckoo parasitism on the hoopoe. However, according to the blocking model (Britton et al. 2007), it could only evolve if efficient nestling discrimination has not evolved. Furthermore, the great spotted cuckoo is non-killer brood parasite, giving the possibility to foster parents to observe both the parasite and their own nestlings (both species look quite different from each other). This comparison when they share the same nest and later out of the nest, may be an important cue to favor nestling and/or fledgling discrimination (Davies and Brooke 1988; Lotem 1993).
The main aim of this study is to test experimentally these two hypotheses using a population of hoopoes maintained in captivity. We experimentally created mixed broods with hoopoe and cuckoo nestlings and analysed the parental feeding behaviour to nestlings (feeding rate) and fledglings (feeding rate and approach rate). This experimental design allows us to analyse whether adult hoopoes are able to discriminate cuckoo nestlings when they can directly compare them with their own nestlings. With the same objective, we created only-cuckoo-broods, i.e. cuckoo nestlings raised without hoopoes, to analyze the ability of the parents to discriminate cuckoos but without any possibility of comparison.
Our first hypothesis predicts that great spotted cuckoo nestlings introduced in hoopoe nests would be fed at a lower rate (Prediction 1) and would present a higher mortality rate (Prediction 2) than hoopoe nestlings and cuckoo nestlings from only-cuckoo-broods. The second hypothesis predicts that cuckoo fledglings from mixed broods would be fed at a lower rate than hoopoe fledglings and cuckoo fledglings from only-cuckoo-broods (Prediction 3) and that cuckoo fledglings from mixed broods would present a higher mortality rate (Prediction 4) than hoopoe fledglings and cuckoos from only-cuckoo-broods. If adults have the capacity to discriminate cuckoos, we predict that: hoopoe adults would approach to hoopoe fledglings and cuckoo fledglings from only-cuckoo-broods to feed more often than cuckoo fledglings from mixed broods (Prediction 5) and that cuckoo fledglings would be fewer likely to get food than hoopoe fledglings, when they approached the adults (Prediction 6).
Materials and methods
Study area
This study was performed during the 2013 breeding season (March-July) in a population of hoopoes maintained in captivity since 2008. During autumn and winter, hoopoes were maintained, separated by sex, in facilities located at the University of Granada (southern Spain). In spring, the captive pairs were housed in independent cages (3 × 2 × 2 m) installed outdoors in a pine forest in the Hoya de Guadix (37° 21’ N, 003° 05’ W, Granada province). This area is a high-altitude plateau (approx. 1000 m a.s.l.) with cereal crops, groves of almond trees (Prunus amygdalus) and some areas with dispersed holm-oak trees (Quercus rotundifolia) and reforested pine forests. We used 20 cages spaced at 50 m from each other to avoid interactions between pairs and ensure successful breeding. All cages had access to soil and were equipped with a roof that provided shadow, a cork nest box (40 cm x 20 cm x 20 cm, 5.5 cm of hole diameter) and an internal roof that protected feeders from sun and rain. Great spotted cuckoo nestlings were collected from magpie nests found in the surroundings. The great spotted cuckoo is a common species in this area, involved in a high incidence of parasitism on magpie hosts with 56.8% of magpie nests parasitized by the great spotted cuckoo during the period 2008—2012 (Soler et al. 2014a).
Experimental design
Hoopoe breeding pairs were established in March, when one male and one female were randomly paired in each cage. Hoopoes were provided with live food (crickets, fly larvae) and meat (beef heart vitamin-enriched) ad libitum. Cages were visited daily to ensure hoopoes care and maintenance and to record laying date, clutch size, and hatching date. At the same time, we searched for magpie nests during nest-building or egg-laying phases so brood parasitism by the great spotted cuckoo was detected soon to be able to calculate hatching date accurately. Close to hatching, we visited nests daily in order to detect cuckoo hatchlings.
We created two types of experimental nests: mixed broods with one cuckoo and a variable number of hoopoe nestlings (from 1 to 5 hoopoe nestlings); and only-cuckoo-broods with one or two cuckoo nestlings. The variable number of hoopoe nestlings is within the natural range of brood sizes in the wild (Martín-Vivaldi et al. 1999). In mixed broods, cuckoo nestlings were introduced 1 to 2 days younger than the first hatched hoopoe nestling. This allowed both species to reach a similar weight at the start of the nestling period, in order to ensure survival of nestlings of both species until fledging. Cuckoo nestlings are larger than hoopoe nestlings, reaching an average weight at hatching of 7.8 g compared to 3.5 g for hoopoe nestlings (Soler and Soler 1991; Hildebrandt and Schaub 2018). At the end of the nestling period, this difference between species is greater (the average weight is 133.7 g in cuckoo nestlings; and 69.6 g in hoopoe nestlings Soler and Soler 1991; Hildebrandt and Schaub 2018)). Furthermore, the introduction of cuckoo nestlings younger than hoopoes matches the nesting period of both species, since cuckoos spend 19–25 days and hoopoes 27.1 days in the nest (Soler and Soler 1991; Martín-Vivaldi et al. 1999). In only-cuckoo-broods, cuckoo hatchlings 1- or 2-days-old were introduced in hoopoe clutches with a total hatching failure. The viability of hoopoe eggs was monitored using an Egg Buddy Digital Heart Monitor (Avitronics, UK). This allowed us to introduce the cuckoo nestlings on the expected hatching date of the hoopoe eggs, ensuring acceptance by the hoopoe female. We removed failed hoopoe eggs after nestling introduction. The reason for using failed hoopoe clutches to obtain only-cuckoo-broods and keeping all hoopoe nestlings in mixed broods follows ethical considerations. On the one hand, we could not move hoopoe nestlings to other nests due to limitations in the number of cages and lack of synchrony in laying date between hoopoe nests. On the other hand, we were not going to sacrifice any hoopoe nestling in the experimental procedure. Cuckoo nestlings were transported in an artificial cotton nest and kept at a temperature between 25 and 30 °C (for further details see Ibáñez-Álamo et al. 2012). No cuckoo died during the transport process. We created 8 mixed broods and 7 only-cuckoo-broods (4 broods with two cuckoo nestlings and 3 broods with only one cuckoo nestling).
We analyzed parental feeding behavior during the nestling period by video recording into nest boxes, and during the fledgling period by direct observations of fledglings in the cage. Nest boxes were video-recorded using micro-cameras (KPC-S500, eSentia Systems Inc., Baton Rouge, LA, USA) connected to video recorders. Further details on the filming procedure can be found in Martín-Gálvez et al. (2011). The observations of fledglings were performed using a hide located about three meters from the cage.
During the nestling period, both mixed and only-cuckoo-broods were filmed once when cuckoo nestlings were between 13 and 18 days old. We considered this range because in the great spotted cuckoo the feathers appear in the majority of the quills between days 12 and 13 (Soler and Soler 1991) and the crest is already developing in the hoopoe by day 14 (Kristin 2001). Therefore, at these ages the differences in development and plumage color between the two species would be more visible, making recognition easier. Recordings lasted approximately two hours and a half and started half an hour after sunrise (the most active period of adult hoopoes) and after the daily food provision to the cage. Information containing age and weight of the nestlings (cuckoos and hoopoes) as well as brood size before video-recordings is provided in Supplementary Table 1. In mixed broods with more than one hoopoe nestling, hoopoe nestlings were randomly marked individually with blue points on the crown with permanent marker (except one nestling that remained unmarked) in order to identify the individual fed in each food provisioning event. There is no reason to expect that the presence of the blue dot might affect parental preference for a hoopoe nestling. Therefore, we decided not to mark the cuckoo which is easily distinguished from hoopoes. In only-cuckoo-broods with two cuckoo nestlings, cuckoo nestlings were distinguishable from each other by size, so we did not mark them either. The parental food delivery was filmed in 8 nest boxes of mixed broods and 6 of only-cuckoo-broods (one cuckoo from a single only-cuckoo-brood died a few days after the beginning of the experiment). We lost some video recordings because one of the videorecorders failed during experimental procedures, leaving us with 5 recordings of mixed broods and 5 of only-cuckoo-broods (Supplementary Table 3). The feeding rate to each nestling (feedings per hour) was calculated as the ratio of feedings events per recording hours.
During the fledgling period, the observations started on the first day that all individuals were outside the nest. The observations lasted approximately two hours and were performed half an hour after sunrise, after the daily food provision to the cage. In mixed broods, the cuckoo fledgling usually left the nest box a few days before the hoopoe fledglings, and hoopoe fledglings left the nest boxes depending on their age, starting from the oldest to youngest nestlings in consecutive days. In cuckoo broods with two fledglings, both individuals left the nest box at the same time.
In each feeding event, we annotated the identity of the fledgling. In mixed broods, we marked the tarsus of the hoopoe fledglings with a red and blue permanent marker to distinguish them from each other. However, it was not always possible to assign feedings to particular hoopoe fledglings in mixed broods due to the high speed of the feeding events. Therefore, the feeding rate to hoopoes in a family was calculated as the number of feedings to all hoopoe fledglings divided by the number of hoopoe fledglings and by the number of hours. In only-cuckoo-broods, we distinguished one fledgling from another by its size and position in the cage. The feeding rate per cuckoo was calculated in a similar way in cases where two cuckoo fledglings were in the cage. The number of observations per nest varied among nests with a range of 1–4 observations (Supplementary Table 2), so we averaged feeding rates of the different observations of nests. Information containing age of the fledglings (cuckoos and hoopoes) as well as brood size during each observation event is provided in Supplementary Table 2.
We also annotated the approaching strategy of parents and fledglings. The adult approach rate is a subsample of the feeding rate in which an adult hoopoe approached the fledgling and fed it per hour. Fledgling approach rate is the number of times that hoopoe/cuckoo fledglings approached an adult hoopoe begging for food per hour. In this sense, we also distinguished between successful (when fledglings approached adults and were fed) from unsuccessful (when fledglings approached adults and were not fed) approaches.
In the fledgling phase we observed only six mixed broods since in one case the cuckoo died the day after leaving the nest and, in another, the two hoopoe fledglings died and only the cuckoo fledgling survived. In only-cuckoo-broods, feeding behavior was observed in five broods since one brood was lost to predation by a fox (Vulpes vulpes) entering the cage by burrowing under the wire mesh. This incident happened before any observation could be performed (Supplementary Table 3).
It was not possible to record data blind because our study involved focal animals in the field.
The period of time during which the cuckoo fledglings were observed to record their survival lasted from day they left the nest box until they reached the post-fledgling independence (when they could feed themselves). The post-fledgling dependence period ranged from 40 to 64 days which is within the range found by Soler et al. (1994) in the wild (25–59 days).
We released the surviving cuckoos in the area of Guadix at the end of July, since the fledglings usually leave the breeding area in the second week of August, to start their migration towards the wintering areas in Subsaharian Africa (Soler et al. 1994).
Statistical analyses
Feeding rate and approach rate fitted a Gaussian distribution after log transformation (Kolmogorov–Smirnov P > 0.20). Therefore, any reference to feeding rate or approach rate refers to the log-transformed variable. In all cases, all dependent variables were homoscedastic (F < 1.61; p > 0.232), validating the use of parametric tests.
In the nestling and fledgling periods and for mixed broods, we used a General Linear Mixed Model (GLMM) exploring differences in feeding rate (dependent variable) between hoopoe and cuckoo nestlings (species as a fixed factor). Nest identity was included as a random effect. In addition, we explored if cuckoos received different feeding rates (dependent variable) depending on their experimental broods (fixed factor: cuckoos raised in mixed broods versus cuckoos raised in only-cuckoo-broods) and brood size as a covariate by means of a General Linear Model (GLM).
To test adult approach rate in mixed broods in the fledgling period, we used a GLMM where the adult approach rate was used as a dependent variable, species as a fixed factor and nest identity as a random effect. To test whether hoopoe adults approached cuckoo fledglings more often when they are raised in mixed broods compared to only-cuckoo-broods, we used a GLM where adult approach rate was used as a dependent variable, the type of experimental brood as a fixed factor and brood size as a covariate.
In order to establish whether cuckoo fledglings were fewer likely to be fed than hoopoe fledglings when they approached the adults, we used a GLMM, where the fledgling approach rate (dependent variable) was calculated separately for successful and unsuccessful approaches (fixed factor) for each species (fixed factor) and controlled by nest identity as a random effect.
To analyze the survival of the cuckoo, we considered that a nestling survived the nestling period when it left the nest box (0 = died before left the nest; 1 = successfully left the nest). Similarly, fledgling survival is the survival since the moment they left the nest until the end of the dependency period (40–64 days after fledging; 0 = died; 1 = survived). To analyze the probability of survival in mixed broods in both the nestling period and the fledgling period, we used a Generalized Linear Model (GLZ), where the probability of survival (binomially distributed response variable) depended of the species identity (explanatory variable; fixed factor) and the nest identity was included as a random effect. In a second model, we used a Generalized Linear Model (GLZ), where the probability of survival (binomially distributed response variable) of the cuckoo differed between type of experimental broods (raised in mixed or only-cuckoo-broods; fixed factor) and the brood size was included as a covariate both in the nestling period and in the fledgling period. Statistical analyses were performed in STATISTICA 12.0 (Statsoft Inc., OK, USA).
Results
Nestling period
In mixed broods, feeding rate was similar between cuckoo and hoopoe nestlings (species, F1,11 = 0.76, p = 0.403; nest identity, F4,11 = 8.78, p = 0.001; Fig. 1a).
Comparisons of feeding rates by adult hoopoes to: (a) hoopoe (Upupa epops) and great spotted cuckoo (Clamator glandarius) nestlings raised in mixed broods; and (b) nestlings of great spotted cuckoo raised in mixed broods and in only-cuckoo-broods. The feeding rate of each nestling was calculated as the logarithm of the number of feedings they received divided by the recorded hours. Means ± standard errors are shown
The feeding rate of the cuckoo nestlings was similar when reared in only-cuckoo-broods or in mixed broods (experimental nest, F1,10 = 0.73, p = 0.412; brood size, F1,10 = 2.99, p = 0.114; Fig. 1b).
Fledgling period
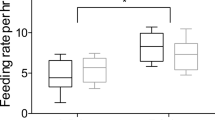
In mixed broods, cuckoo fledglings received fewer feedings than hoopoe fledglings (species, F1,5 = 6.70, p = 0.048; nest identity, F5,5 = 0.90, p = 0.543; Fig. 2a). Moreover, cuckoo fledglings from mixed broods received fewer feedings than those from only-cuckoo-broods (experimental broods; F1,8 = 11.73, p = 0.009; brood size, F1,8 = 3.55, p = 0.096; Fig. 2b).
Comparisons of feeding rates by adult hoopoes (a) to hoopoe (Upupa epops) and great spotted cuckoo (Clamator glandarius) fledglings in mixed broods (raised in the same cage); and (b) to fledglings of great spotted cuckoo raised in mixed broods and in only-cuckoo-broods (cages only with cuckoo fledglings). The feeding rate was calculated as the logarithm of the number of feedings to all hoopoe fledglings divided by the number of hoopoe fledglings and by the number of observation hours. In only-cuckoo-broods, the feeding rate per cuckoo was calculated in a similar way. Means ± standard errors are shown
Approaching preferences
In mixed broods, hoopoe adults approached more often to feed hoopoe fledglings than cuckoo fledglings (species, F1,5 = 28.46, p = 0.003; nest identity, F5,5 = 2.59, p = 0.159; Fig. 3a). In addition, hoopoe adults approached more often to feed cuckoo fledglings from only-cuckoo-broods than cuckoo fledglings from mixed broods (experimental broods, F1,8 = 6.28, p = 0.036; brood size, F1,8 = 0.002, p = 0.969; Fig. 3b).
Adult approach rates taking into account only when adult hoopoes approached to (a) great spotted cuckoo (Clamator glandarius) and hoopoe (Upupa epops) fledglings raised in mixed broods, and (b) great spotted cuckoo fledglings raised in mixed and only-cuckoo-broods. The adult approach rate was calculated as the logarithm of the number of feedings to all hoopoe fledglings divided by the number of hoopoe fledglings and by the number of observations. In only-cuckoo-broods, the adult approach rate per cuckoo was calculated in a similar way. Means ± standard errors are shown
In mixed broods, differences between successful and unsuccessful fledgling approach rate were similar for both species (see interaction term: successful/unsuccessful approaches, F1,15 = 4.06, p = 0.062; species, F1,15 = 0.49, p = 0.495; interaction term, F1,15 = 2.48, p = 0.136; nest identity, F5,15 = 0.51, p = 0.676; Fig. 4). However, unsuccessful approach rate in cuckoo fledglings was significantly higher than their successful approach rate (LSD, p = 0.023; Fig. 4). In contrast, no differences were found between successful and unsuccessful approach rate in fledgling hoopoes (LSD, p = 0.759; Fig. 4).
Comparisons of fledgling approach rates of hoopoe fledgling (Upupa epops – in red) and great spotted cuckoo fledgling (Clamator glandarius – in green) in mixed broods, distinguishing successful approach events (when fledglings approached adults and were fed) from unsuccessful approach events (when fledglings approached adults and were not fed). Fledgling approach rate was calculated as the logarithm of the number of times hoopoe/cuckoo fledglings approached an adult begging for food per hour (regardless of whether or not they were fed). Means ± standard errors are shown
Survival
In mixed broods, the survival of hoopoe and cuckoo nestlings during the nestling period was similar (species, x21 = 1.90, p = 0.168; nest identity, x27 = 0.01, p = 0.999). Nevertheless, survival was significantly lower for cuckoos than for hoopoes in the fledgling period (species, χ21 = 10.57, p = 0.001; nest identity, x27 = 0.01, p = 0.999; Fig. 5).
Probability of survival of hoopoes (Upupa epops – in red) and great spotted cuckoo (Clamator glandarius) both raised in mixed broods (nestling cuckoo raised with hoopoe nestlings -in green) and in only-cuckoo-broods (cuckoos raised alone in hoopoe nests – in blue), during the nestling and the fledgling periods. Means ± standard errors are shown
The survival of cuckoo fledglings raised in mixed or only-cuckoo-broods was similar during the nestling period (experimental brood, x21 = 0.29, p = 0.593; brood size, x21 = 0.58, p = 0.445), and during the fledgling period (experimental brood, x21 = 0.63, p = 0.425; brood size, x21 = 0.86, p = 0.352).
Nestling survival of the great spotted cuckoo
Eighty-eight percent of cuckoos nestlings (15 up to 17) survived the nestling phase, successfully leaving the nestbox.
Survival of great spotted cuckoo and hoopoe fledglings
Survival of cuckoo fledglings (those fledglings that reached post-fledgling independence), including both experimental broods, was 6 out 15 (40%). Separately, 3 out of 7 cuckoo fledglings survived in only-cuckoo-broods (42.86%), while 3 out of 8 cuckoo fledglings survived in mixed broods (37.50%). Survival of hoopoe fledglings was 19 out 20 (95%).
Weight and age of great spotted cuckoo fledglings at death
All the cuckoo fledglings that died showed signs of undernourishment, weighing at death from 60 to 103 g, although this information was only collected for 5 cuckoos out of 9 (Supplementary Table 3). Cuckoo fledglings died between 28 and 62 days of age (n = 9; Supplementary Table 3).
Discussion
Brood parasitic cuckoos use only an extremely low proportion of available potential host species (Davies 2000; Martín-Vivaldi et al. 2013). Many of these species present a high ability of egg recognition and rejection, which preclude successful parasitism. However, a high percentage of potential host species lack egg rejection (38.5%: Soler 2014). Why then are they not parasitized? A possible answer would be that hosts have evolved an efficient defense at some of the other stages of the breeding cycle: before laying, or during nestling or fledgling periods (Feeney et al. 2014; Soler 2014, 2017c). However, the existence of defenses during these stages has been studied only in a few potential host species. In this study, we have tested experimentally whether the hoopoe, a potential host species of the great spotted cuckoo without egg rejection ability, presents defenses after the egg stage. By parasitizing hoopoe nests with great spotted cuckoo nestlings and recording brood feeding by adults, we have found an absence of defenses during the nestling period but discrimination and undernourishment of the great spotted cuckoo during the fledgling period.
During the nestling period, hoopoe adults fed great spotted cuckoo and hoopoe nestlings raised in the same nest at the same rate, and the survival of hoopoe and cuckoo nestlings was similar. These results suggest that hoopoes did not discriminate their own nestlings from experimental ones.
Host defenses at the nestling stage have been reported in several potential host species (Langmore et al. 2003; Sato et al. 2010; Tokue and Ueda 2010; Attisano et al. 2021); (reviewed in Grim 2006, 2017; Soler 2009). In several brood parasite-host systems, some brood parasites have evolved adaptations to counteract these host defenses (De Mársico et al. 2017). The evolution of any host defense is related to the absence of other efficient host defenses at earlier stages (Britton et al. 2007; Soler 2014; Grim 2017). Thus, in the hoopoe, a potential host species lacking egg-rejection defenses, the existence of nestling discrimination could be expected. However, we have found that the hoopoe does not show nestling discrimination, even when having the possibility to compare between the parasite and their own host nestlings (experimental mixed broods), which has been suggested as a crucial clue for nestling recognition (Davies and Brooke 1988; Lotem 1993). In contrast, the fan-tailed gerygone “Gerygone flavolateralis” and its specialist brood parasite, the shining bronze cuckoo “Chalcites lucidus” in New Caledonia is an example of a host that discriminates cuckoo nestlings without direct comparison with own nestlings (Sato et al. 2015; Attisano et al. 2021) Although nestling discrimination is more common among potential host species than previously suspected, this behavior is absent in egg- and fledgling-rejecters (Feeney 2017; Grim 2017), as it would be the case of the hoopoe (this study). This result is not surprising considering that absence of effective defenses at earlier stages of the nestling cycle implies stronger selection pressures for the evolution of defenses at a later stage (Britton et al. 2007; Feeney et al. 2012, 2014; Feeney 2017).
During the fledgling period, cuckoo fledglings reared in mixed broods received fewer feedings and died more frequently than hoopoe fledglings. Cuckoo fledglings died between 28 and 62 days of age, within the range in which they are dependent on their host parents (Soler et al. 1994). All the cuckoo fledglings that died were extremely thin, weighing at death from 60 to 103 g. This range of weights is very low for a fledgling cuckoo, since they can weigh up to 133.7 g at the end of the nestling period (Soler and Soler 1991) suggesting the cause of death was undernourishment. Indeed, hoopoe adults approached to feed cuckoo fledglings fewer frequently than to hoopoe fledglings. When it was the fledglings that approached the hoopoe adults begging for food, no differences were found between cuckoo and hoopoe fledglings in successful and unsuccessful approaches (i.e. approaching an adult and received or not received food respectively). However, most of the approaches of cuckoo fledglings to adults were unsuccessful, with the fledgling not receiving food. Hoopoe fledglings, however, showed not difference in the number of successful and unsuccessful approaches (see Fig. 4). Thus, our experimental study points out that hoopoe adults feed more often their own fledglings than parasitic cuckoos, which could indicate recognition and discrimination of the brood parasite in the fledgling stage. As far as we know, the hoopoe is the third potential host species showing fledgling discrimination. The relationship between foster parents and fledgling brood parasites has been studied in detail in only two brood parasite-host systems (De Mársico et al. 2017). This lack of studies at the post-fledging phase is expected because this is the least studied stage in the avian nesting cycle (Gruebler and Naef-Daenzer 2010; Matthysen et al. 2010). The best studied brood parasite, the common cuckoo only provides anecdotal information for this stage (Davies 2000; Tyller et al. 2018). Regarding other well studied brood parasites, in the brown-headed cowbird (Molothrus ater), parasitic fledglings are fed at a higher rate than host fledglings, which were only rarely fed, provoking a much lower survival rate of host fledglings, implying no recognition of parasites (Woodward 1983). In contrast, a recent paper by Jones et al. (2022) failed to support substantial post-fledging costs of brown-headed cowbird parasitism. The baywing (Agelaioides badius) is parasitized by two brood parasites, the specialist screaming cowbird (Molothrus rufoaxillaris) and the generalist shiny cowbird (Molothrus bonariensis). In a detailed study of this system, De Mársico et al. (2012, 2017) found that the baywing is willing to feed fledglings of the specialist parasite, which mimic both visually and vocally host fledglings, but refuse to feed fledglings of the generalist brood parasite, which does not mimic host fledglings. The relationships between foster parents and fledgling brood parasites have also been studied in detail in the great spotted cuckoo-magpie host system. The magpie feeds parasite fledglings when they have been reared in only-cuckoo-broods but were progressively more reluctant to feed parasite fledglings reared in mixed broods, implying parasite recognition (Soler et al. 2014a).
Both the cowbird and the great spotted cuckoo are non-killer brood parasites, giving the baywing and the magpie the possibility to compare the parasite and their own nestlings sharing the nest and later out of the nest, which may be an important cue to favor nestling and/or fledgling discrimination. To test this possibility in the hoopoe, we created both only-cuckoo and mixed broods in our experimental design. We found that cuckoo fledglings from mixed broods received fewer feedings than cuckoo fledglings from only-cuckoo-broods. In addition, as it occurred in the magpie (Soler et al. 2014b), the hoopoe approached more frequently to feed cuckoo fledglings from only-cuckoo-broods than to cuckoo fledglings from mixed ones. These results suggest that the presence of hoopoe fledglings helps parents in the recognition of the cuckoo fledgling in mixed broods.
Despite the difference found in willingness of parents to feed cuckoos in the two situations (reared alone or in mixed broods), the mortality was not different between both treatments, but high and significantly higher than that of hoopoe fledglings. This may indicate that, even the higher feeding rate received by cuckoo fledglings in only-cuckoo-broods did not satisfy the needs of the parasite. The hoopoes is frequently able to feed up to six hoopoe nestlings (Hildebrandt and Schaub 2018), the brood thus summing up to 450 g (6 × 75 g), and therefore should be able to rear a cuckoo nestling despite its bigger size (134 g), if the hoopoe was motivated to do so. This should be the case especially in our captivity conditions with food ad libitum. Therefore, our results suggest that, in both experimental situations, adult hoopoes were feeding cuckoo fledglings at lower rates than expected if they did not recognize them as parasites. This implies that contrary to what has been suggested (Fraga 1998; De Mársico et al. 2012, 2017; Soler et al. 2014a, b) and according to recent studies of hosts of the bronze-cuckoo (Sato et al. 2015; Attisano et al. 2018), the presence of own host fledglings for comparison is not necessary for the evolution of fledgling discrimination.
Our main conclusion is that the hoopoe would be able to discriminate parasite fledglings, even in the absence of their own fledglings for comparison. This phenomenon, inconceivable only 20 years ago (Davies 2000), is in agreement with current theory which suggests that an efficient host defense can evolve at any stage of the breeding cycle, driving the outcome of the long-term coevolution of both brood parasite and host (Soler 2014, 2017a).
The existence of discrimination ability of cuckoo fledglings by the hoopoe would indicate that this species has been parasitized at least in the past. However, the success of cuckoo fledglings in our experimentally parasitized hoopoe broods (40%) is not so low to conclude that it has been abandoned as a host by the development of defenses, as predicted by the coevolutionary alternation hypothesis (Davies and Brooke 1989a; Nuismer and Thompson 2006). Species with intermediate levels of defenses (around 50%) have been traditionally considered accepters (Davies 2000; Martín-Vivaldi et al. 2013) and therefore, the fledgling recognition ability found in the hoopoe (60%) cannot be the only reason for its rare usage as a host by the great spotted cuckoo.
It is known that host life-history variables that reduce the probability of parasitism, thereby reducing selection pressure due to parasitism, could explain low or intermediate levels of defense. It is the case of hole nesting (Aviles et al. 2005; Thomson et al. 2016), habitats without vantage points for cuckoos (Roskaft et al. 2002; Martín-Vivaldi et al. 2013), or ground nesting (Martín-Vivaldi et al. 2013). In these cases, low parasitism rates are caused by the increased difficulty of finding nests for the parasites (no perches for observation of hosts behaviors or ground nesting) or of laying the egg within nests found (because of the small size of some nest entrances in hole nesting species) (Moreras et al. 2021). The hoopoe combines both kinds of difficulties for a laying cuckoo female. First, the hoopoe does not build a nest, does not transport nest materials to the hole selected and, therefore, there is almost no clue on the place where a pair will lay eggs before eggs can be found within a particular hole. Male visits with food to the nest are only frequent after eggs hatch and, so, it is very difficult to find nests before hatching (Martín-Vivaldi et al. 1999). Second, some nests are in holes with a very narrow opening for a female cuckoo to enter or a cuckoo nestling to fledge. These two life history traits of the hoopoe may explain why the parasite pressure has not been so high to cause the evolution of higher levels of fledgling recognition. In summary, the hoopoe is a suitable but not heavily parasitized host in which only about 40% of nests found and parasitized by the great spotted cuckoo, would be successful in producing surviving cuckoo fledglings. The difficulty of finding enough usable nests would have made that this species only a secondary host, explaining low levels of defenses.
Data availability
The datasets generated during and/or analysed during the current study are available in the DIGIBUB repository, https://hdl.handle.net/10481/77885
References
Amor-García A, Palacios-Colomer T, Basanta-Reyes L (2020) Primera cita, documentada gráficamente, sobre Abubilla (Upupa epops Linnaeus, 1758) cebando un volantón de Críalo europeo (Clamator glandarius Linnaeus, 1758). https://doi.org/10.13140/RG.2.2.30795.00804
Attisano A, Sato NJ, Tanaka KD, Okahisa Y, Kuehn R, Gula R, Ueda K, Theuerkauf J (2018) Visual discrimination of polymorphic nestlings in a cuckoo-host system. Sci Rep 8:10359
Attisano A, Sato NJ, Tanaka KD, Okahisa Y, Ueda K, Gula R, Theuerkauf J (2021) Discrimination and ejection of eggs and nestlings by the fan-tailed gerygone from New Caledonia. Curr Zool 67:653–663
Aviles JM, Rutila J, Møller AP (2005) Should the redstart Phoenicurus phoenicurus accept or reject cuckoo Cuculus canorus eggs? Behav Ecol Sociobiol 58:608–617. https://doi.org/10.1007/s00265-005-0941-7
Bolopo D, Canestrari D, Roldán M, Baglione V, Soler M (2015) High begging intensity of great spotted cuckoo nestlings favours larger-size crow nest mates. Behav Ecol Sociobiol 69:873–882. https://doi.org/10.1007/s00265-015-1895-z
Britton NF, Planque R, Franks NR (2007) Evolution of defence portfolios in exploiter-victim systems. Bull Math Biol 69:957–988. https://doi.org/10.1007/s11538-006-9178-5
Brooke M de L, Davies NB (1988) Egg mimicry by cukoos Cuculus canorus in relation to discrimination by hosts. Nature 335:630–632
Cramp S (1998) The complete birds of the Western Palearctic on CD-ROM. Oxford: Software Optimedia. Oxford University Press, Oxford
Davies NB (2000) Cuckoos, cowbirds and other cheats. T & AD Poyser, London
Davies NB (2011) Cuckoo adaptations: trickery and tuning. J Zool 284:1–14. https://doi.org/10.1111/j.1469-7998.2011.00810.x
Davies NB, Brooke M de L (1988) Cuckoos versus reed warblers: adaptations and counteradaptations. Anim Behav 36:262–284. https://doi.org/10.1016/s0003-3472(88)80269-0
Davies NB, Brooke M de L (1989a) An experimental-study of co-evolution between the cuckoo, Cuculus-canorus, and its hosts. 1 Host egg discrimination. J Anim Ecol 58:207–224. https://doi.org/10.2307/4995
Davies NB, Brooke M de L (1989b) An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J Anim Ecol 58:225–236. https://doi.org/10.2307/4996
De Mársico MC, Fiorini VD, Tuero DT, Gloag R, Ursino CA, Reboreda JC (2017) Parasite adaptations during the nestling and fledgling stages. In: Soler M (ed) Avian Brood Parasitism: Behaviour, Ecology, Evolution and Coevolution. Springer International, Cham, pp 557–574
De Mársico MC, Gantchoff MG, Reboreda JC (2012) Host-parasite coevolution beyond the nestling stage? Mimicry of host fledglings by the specialist screaming cowbird. Proc R Soc Lond B 279:3401–3408. https://doi.org/10.1098/rspb.2012.0612
Díaz-Lora S, Martín-Vivaldi M, Juárez García-Pelayo N, AzcárateGarcía M, Rodríguez-Ruano SM, Martínez-Bueno M, Soler JJ (2019) Experimental old nest material predicts hoopoe Upupa epops eggshell and uropygial gland microbiota. J Avian Biol 50:e02083. https://doi.org/10.1111/jav.02083
Díaz-Lora S, Pérez-Contreras T, Azcárate-García M, Manuel Peralta-Sánchez J, Martínez-Bueno M, Soler JJ, Martín-Vivaldi M (2021) Cosmetic coloration of cross-fostered eggs affects paternal investment in the hoopoe (Upupa epops). Proc R Soc B 288:20203174. https://doi.org/10.1098/rspb.2020.3174
Erritzøe J, Mann CF, Brammer F, Fuller RA (2012) Cuckoos of the World. Bloomsbury Publishing, London
Feeney WE (2017) Evidence of adaptations and counter-adaptations before the parasite lays its egg: The frontline of the arms race. In: Soler M (ed) Avian Brood Parasitism: Behaviour, Ecology, Evolution and Coevolution. Springer International, Cham, pp 307–324
Feeney WE, Welbergen JA, Langmore NE (2012) The frontline of avian brood parasite-host coevolution. Anim Behav 84:3–12. https://doi.org/10.1016/j.anbehav.2012.04.011
Feeney WE, Welbergen JA, Langmore NE (2014) Advances in the study of coevolution between avian brood parasites and their hosts. Annu Rev Ecol Syst 45:227–246. https://doi.org/10.1146/annurev-ecolsys-120213-091603
Fraga RM (1998) Interactions of the parasitic screaming and shiny cowbirds (Molothrus rufoaxillaris and M. bonariensis) with a shared host, the bay-winged cowbird (M. badius). In: Rothstein SI, Robinson SK (eds) Parasitic Birds and Their Hosts. Studies in Coevolution. Oxford University Press, New York, pp 173–193
Grim T (2006) The evolution of nestling discrimination by hosts of parasitic birds: why is rejection so rare? Evol Ecol Res 8:785–802
Grim T (2017) Host defences against brood parasite nestlings: Theoretical expectations and empirical evidence. In: Soler M (ed) Avian Brood Parasitism: Behaviour, Ecology, Evolution and Coevolution. Springer International, Cham, pp 539–556
Gruebler MU, Naef-Daenzer B (2010) Survival benefits of post-fledging care: experimental approach to a critical part of avian reproductive strategies. J Anim Ecol 79:334–341. https://doi.org/10.1111/j.1365-2656.2009.01650.x
Hildebrandt B, Schaub M (2018) The effects of hatching asynchrony on growth and mortality patterns in Eurasian Hoopoe Upupa epops nestlings. Ibis 160:145–157. https://doi.org/10.1111/ibi.12529
Hoffmann J, Postma E, Schaub M (2015) Factors influencing double brooding in Eurasian Hoopoes Upupa epops. Ibis 157:17–30. https://doi.org/10.1111/ibi.12188
Ibáñez-Álamo JD, Arco L, Soler M (2012) Experimental evidence for a predation cost of begging using active nests and real chicks. J Ornithol 153:801–807. https://doi.org/10.1007/s10336-011-0797-8
Johnsgard PA (1997) The avian brood parasites: Deception at the nest. Oxford University Press, New York
Jones TM, Di Giovanni AJ, Hauber ME, Ward MP (2022) Ontogenetic effects of brood parasitism by the Brown-headed Cowbird on host offspring. Ecology 104:e3925. https://doi.org/10.1002/ecy.3925
Kristin A (2001) Family Upupidae (Hoopoe). In: delHoyo J, Elliott H, Sargatal J (eds) Handbook of the Birds of the World, vol. 6. Mousebirds to Hornbills. Lynx Edicions, Barcelona, pp 396–411
Langmore NE, Hunt S, Kilner RM (2003) Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature 422:157–160. https://doi.org/10.1038/nature01460
Löhrl H (1979) Untersuchungen am Kuckuck, Cuculus canorus (Biologie, Ethologie und Morphologie). J Ornithol 120:139–173. https://doi.org/10.1007/BF01642995
Lotem A (1993) Learning to recognize nestlings is maladaptive for cuckoo Cuculus-canorus host. Nature 362:743–745. https://doi.org/10.1038/362743a0
Macías-Sánchez E, Martínez JG, Avilés JM, Soler M (2013) Sexual differences in colour and size in the Great Spotted Cuckoo Clamator glandarius. Ibis 155:605–610. https://doi.org/10.1111/ibi.12045
Martín-Gálvez D, Pérez-Contreras T, Soler M, Soler JJ (2011) Benefits associated with escalated begging behaviour of black-billed magpie nestlings overcompensate the associated energetic costs. J Exp Biol 214:1463–1472. https://doi.org/10.1242/jeb.050153
Martín-Vivaldi M, Palomino JJ, Soler M, Soler JJ (1999) Determinants of reproductive success in the Hoopoe Upupa epops, a hole-nesting non-passerine bird with asynchronous hatching. Bird Study 46:205–216. https://doi.org/10.1080/00063659909461132
Martín-Vivaldi M, Ruiz-Rodríguez M, Méndez M, Soler JJ (2006) Relative importance of factors affecting nestling immune response differs between junior and senior nestlings within broods of hoopoes Upupa epops. J Avian Biol 37:467–476. https://doi.org/10.1111/j.0908-8857.2006.03660.x
Martín-Vivaldi M, Ruiz-Rodríguez M, Soler JJ, Peralta-Sánchez JM, Méndez M, Valdivia E, Martín-Platero AM, Martínez-Bueno M (2009) Seasonal, sexual and developmental differences in hoopoe Upupa epops preen gland morphology and secretions: evidence for a role of bacteria. J Avian Biol 40:191–205. https://doi.org/10.1111/j.1600-048X.2009.04393.x
Martín-Vivaldi M, Soler JJ, Møller AP, Pérez-Contreras T, Soler M (2013) The importance of nest-site and habitat in egg recognition ability of potential hosts of the common cuckoo Cuculus canorus. Ibis 155:140–155. https://doi.org/10.1111/ibi.12000
Matthysen E, Van Overveld T, Van de Casteele T, Adriaensen F (2010) Family movements before independence influence natal dispersal in a territorial songbird. Oecologia 162:591–597. https://doi.org/10.1007/s00442-009-1483-x
Moreras A, Tolvanen J, Morosinotto C, Bussiere E, Forsman J, Thomson RL (2021) Choice of nest attributes as a frontline defense against brood parasitism. Behav Ecol 32:1285–1295. https://doi.org/10.1093/beheco/arab095
Moskát C, Hauber ME, Louder MIM (2017) The evolution of nest sharing and nest mate killing strategies in brood parasites. In: Soler M (ed) Avian Brood Parasitism: Behaviour, Ecology, Evolution and Coevolution. Springer International, Cham, pp 475–492
Nuismer SL, Thompson JN (2006) Coevolutionary alternation in antagonistic interactions. Evolution 60:2207–2217
Roldán M, Martín-Gálvez D, Rodríguez J, Soler M (2013a) Breeding biology and fledgling survival in a Carrion Crow Corvus corone population of southern Spain: a comparison of group and pair breeder. Acta Ornithol 48:221–235. https://doi.org/10.3161/000164513x678865
Roldán M, Soler M (2011) Parental-care parasitism: how do unrelated offspring attain acceptance by foster parents? Behav Ecol 22:679–691. https://doi.org/10.1093/beheco/arr041
Roldán M, Soler M, Márquez R, Soler JJ (2013b) The vocal begging display of great spotted cuckoo Clamator glandarius nestlings in nests of its two main host species: genetic differences or developmental plasticity? Ibis 155:867–876. https://doi.org/10.1111/ibi.12088
Roskaft E, Moksnes A, Stokke BG, Moskat C, Honza M (2002) The spatial habitat structure of host populations explains the pattern of rejection behavior in hosts and parasitic adaptations in cuckoos. Behav Ecol 13:163–168. https://doi.org/10.1093/beheco/13.2.163
Ruiz-Rodríguez M, Martínez-Bueno M, Martín-Vivaldi M, Valdivia E, Soler JJ (2013) Bacteriocins with a broader antimicrobial spectrum prevail in enterococcal symbionts isolated from the hoopoe’s uropygial gland. FEMS Microbiol Ecol 85:495–502. https://doi.org/10.1111/1574-6941.12138
Ryser S, Guillod N, Bottini C, Arlettaz R, Jacot A (2016) Sex-specific food provisioning patterns by parents in the asynchronously hatching European Hoopoe. Anim Behav 117:15–20. https://doi.org/10.1016/j.anbehav.2016.04.015
Sato NJ, Tanaka KD, Okahisa Y, Yamamichi M, Kuehn R, Gula R, Ueda K, Theuerkauf J (2015) Nestling polymorphism in a cuckoo-host system. Curr Biol 25:R1164–R1165
Sato NJ, Tokue K, Noske RA, Mikami OK, Ueda K (2010) Evicting cuckoo nestlings from the nest: a new anti-parasitism behaviour. Biol Lett 6:67–69. https://doi.org/10.1098/rsbl.2009.0540
Soler JJ, Avilés JM, Soler M, Møller AP (2003) Evolution of host egg mimicry in a brood parasite, the great spotted cuckoo. Biol J Linn Soc 79:551–563. https://doi.org/10.1046/j.1095-8312.2003.00209.x
Soler JJ, Soler M (2000) Brood-parasite interactions between great spotted cuckoos and magpies: a model system for studying coevolutionary relationships. Oecologia 125:309–320. https://doi.org/10.1007/s004420000487
Soler M (1990) Relationships between the great spotted cuckoo Clamator glandarius and its corvid hosts in a recently colonized area. Ornis Scand 21:212–223. https://doi.org/10.2307/3676781
Soler M (2001) Begging behaviour of nestlings and food delivery by parents: the importance of breeding strategy. Acta Ethol 4:59–63
Soler M (2002) Breeding strategy and begging intensity: Influences on food delivery by parents and host selection by parasitic cuckoos. In: Wright J, Leonard ML (eds) Evolution of Begging: Competition, Cooperation and Communication. Kluwer Academic Publisher, New York, pp 413–427
Soler M (2009) Co-evolutionary arms race between brood parasites and their hosts at the nestling stage. J Avian Biol 40:237–240. https://doi.org/10.1111/j.1600-048X.2009.04676.x
Soler M (2014) Long-term coevolution between avian brood parasites and their hosts. Biol Rev 89:688–704. https://doi.org/10.1111/brv.12075
Soler M (ed) (2017) Avian brood parasitism: Behaviour, ecology, evolution and coevolution. Springer International, Cham
Soler M (2017) Begging behaviour, food delivery and food acquisition in nests with brood parasitic nestlings. In: Soler M (ed) Avian Brood Parasitism: Behaviour, Ecology, Evolution and Coevolution. Springer International, Cham, pp 493–515
Soler M (2017) Brood parasitism in birds: A coevolutionary point of view. In: Soler M (ed) Avian Brood Parasitism: Behaviour, Ecology, Evolution and Coevolution. Springer International, Cham, pp 1–19
Soler M, de Neve L, Roncalli G, Macías-Sánchez E, Ibáñez-Álamo JD, Pérez-Contreras T (2014a) Great spotted cuckoo fledglings are disadvantaged by magpie host parents when reared together with magpie nestlings. Behav Ecol Sociobiol 68:333–342. https://doi.org/10.1007/s00265-013-1648-9
Soler M, Møller AP (1990) Duration of sympatry and coevolution between the great spotted cuckoo and its magpie host. Nature 343:748–750. https://doi.org/10.1038/343748a0
Soler M, Palomino JJ, Martinez JG, Soler JJ (1994) Activity, survival, independence and migration of fledgling great spotted cuckoos. Condor 96:802–805. https://doi.org/10.2307/1369485
Soler M, Pérez-Contreras T, Ibáñez-Álamo JD, Roncalli G, Macías-Sanchez E, de Neve L (2014) Great spotted cuckoo fledglings often receive feedings from other magpie adults than their foster parents: Which magpies accept to feed foreign cuckoo fledglings? PLoS One 9:e107412. https://doi.org/10.1371/journal.pone.0107412
Soler M, Soler JJ (1991) Growth and development of great spotted cuckoos and their magpie host. Condor 93:49–54. https://doi.org/10.2307/1368605
Soler M, Soler JJ, Pérez-Contreras T, Martínez JG (2002) Differential reproductive success of great spotted cuckoos Clamator glandarius parasitising magpies Pica pica and carrion crows Corvus corone: the importance of parasitism costs and host defences. Avian Sci 2:25–32
Stokke BG, Moksnes A, Røskaft E (2005) The enigma of imperfect adaptations in hosts of avian brood parasites. Ornithol Sci 4:17–29. https://doi.org/10.2326/osj.4.17
Thomson RL, Tolvanen J, Forsman JT (2016) Cuckoo parasitism in a cavity nesting host: near absent egg-rejection in a northern redstart population under heavy apparent (but low effective) brood parasitism. J Avian Biol 47:363–370. https://doi.org/10.1111/jav.00915
Tokue K, Ueda K (2010) Mangrove gerygones Gerygone laevigaster eject Little bronze-cuckoo Chalcites minutillus hatchlings from parasitized nests. Ibis 152:835–839. https://doi.org/10.1111/j.1474-919X.2010.01056.x
Tyller Z, Kysucan M, Grim T (2018) Postfledging behavior of the Common Cuckoo (Cuculus canorus) attended by the Chaffinch (Fringilla coelebs): a comprehensive approach to study the least-known stage of brood parasite-host coevolution. Wilson J Ornithol 130:536–542. https://doi.org/10.1676/16-223.1
Woodward PW (1983) Behavioral ecology of fledgling brown-headed cowbirds and their hosts. Condor 85:151–163. https://doi.org/10.2307/1367248
Acknowledgements
We are grateful to Jonathan Romero Masegosa, Manuel Soto, Jorge Doña, Alicia Astasio, Elisa García, Rosa María Morales and Natalia Juárez García-Pelayo for their help in caring for captive hoopoes. We would like to thank to Tomás Pérez Contreras of University of Granada for his help in the search of spotted cuckoo nestlings.
Funding
Funding for open access publishing: Universidad de Granada/CBUA This research was funded by Spanish Ministry of Science and Innovation, European funds (FEDER) (CGL2007-61940/BOS, CGL2010-19233-C03-03) and the Junta de Andalucía (P09-RNM- 4557, P18-FR-2215). LA received a contract of technical support staff from the Spanish Ministry of Science and Innovation (Subprogram PTA-MICINN) (Ref. PTA2010-4298-I).
Author information
Authors and Affiliations
Contributions
Conceptualization, MS; methodology, MS, LA and MM-V; validation, MS and LA; formal analysis, LA and JMP-S; investigation, LA, MS and MM-V; resources, MS and MM-V; data curation, LA; writing—original draft preparation, MS, LA; writing—review and editing, MS, LA, JMP-S and MM-V; visualization, LA and JMP-S; supervision, MS; funding acquisition, MS and MM-V All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
We performed the experiment in accordance with relevant Spanish national (Real Decreto 1201/2005, de 10 de octubre) and regional government administration (Dirección General de Gestión del Medio Natural, Junta de Andalucía). Ethical approval for this study was not required. Junta de Andalucía approved the establishment and maintenance of the captive breeding population (Resolución de 14 de abril de 2008) and conceded the permit required (Ref: SGYB/FOA/AFR/CFS) to perform the present research according to Spanish regulations (Resoluciones de 14 de abril de 2008 and 23 de marzo de 2010). Cross-fostering of great spotted cuckoo nestlings and the experimental parasitism of various species, including the hoopoe, was approved by Dirección General de Gestión del Medio Natural, Junta de Andalucía (Ref.: SGMN/GyB/JMIF).
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Communicated by M. Leonard
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arco, L., Peralta-Sánchez, J.M., Martín-Vivaldi, M. et al. Fledgling discrimination in the hoopoe, a potential host species of the great spotted cuckoo. Behav Ecol Sociobiol 77, 61 (2023). https://doi.org/10.1007/s00265-023-03338-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03338-2