Abstract
Sexual selection is a major force shaping morphological and behavioral diversity. Existing theory focuses on courtship display traits such as morphological ornaments whose costs and benefits are assumed be to fixed across individuals’ lifetimes. In contrast, empirically observed displays are often inherently dynamic, as vividly illustrated by the acrobatic dances, loud vocalizations, and vigorous motor displays involved in courtship behavior across a broad range of taxa. One empirically observed form of display flexibility occurs when signalers adjust their courtship investment based on the number of rival signalers. The predictions of established sexual selection theory cannot readily be extended to such displays because display expression varies between courtship events, such that any given display may not reliably reflect signaler quality. We thus lack an understanding of how dynamic displays coevolve with sexual preferences and how signalers should tactically adjust their display investment across multiple courtship opportunities. To address these questions, we extended an established model of the coevolution of a female sexual preference and a male display trait to allow for flexible, dynamic displays. We find that such a display can coevolve with a sexual preference away from their naturally selected optima, though display intensity is a weaker signal of male quality than for non-flexible displays. Furthermore, we find that males evolve to decrease their display investment when displaying alongside more rivals. This research represents a first step towards generalizing the findings of sexual selection theory to account for the ubiquitous dynamism of animal courtship.
Significance statement
Animal courtship displays are typically costly for survival: songs attract predators; dances are exhausting; extravagant plumage is cumbersome. Because of the trade-off between mating benefits and survival costs, displaying individuals often vary their displays across time, courting more intensely when the potential benefit is higher or the cost is lower. Despite the ubiquity of such adjustment in nature, existing theory cannot account for how this flexibility might affect the coevolution of displays with sexual preferences, nor for the patterns of tactical display adjustment that might result, because those models treat displays as static, with fixed costs and benefits. Generalizing a well-studied model of sexual selection, we find that a static display and a flexible display can evolve under similar conditions. Our model predicts that courtship should be less intense when more competitors are present.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual selection is a major force shaping morphological and behavioral diversity (Andersson 1994; Andersson and Simmons 2006). A vast body of theoretical work has uncovered conditions under which sexual preferences can lead to the exaggeration of conspicuous morphological “ornaments” that impose survival costs on the individuals that bear them (Maynard Smith 1985; Kokko et al. 2002; Mead and Arnold 2004; Kuijper et al. 2012; Henshaw et al. 2022). Established models assume these ornaments to be static such that an individual’s display expression changes only on long timescales (e.g., across life stages or through growth), if at all. Yet, empirically observed displays are often inherently dynamic, as vividly illustrated by the acrobatic dances, loud vocalizations, and vigorous motor displays involved in courtship behavior across a broad range of taxa (Byers et al. 2010; Spezie et al. 2022).
In contrast to the ornaments of established sexual selection theory, these dynamic displays vary in their expression both within and between courtship events (Patricelli et al. 2016). The ubiquity of such displays poses a major theoretical challenge because established “good-genes” models of sexual selection rely on a fixed relationship between an individual’s quality and display expression: for every possible individual quality, there exists a unique display value, from which we may determine an associated mating benefit and survival cost. Then a preference for a costly display trait may evolve if the display intensity is an honest signal of quality such that individuals of higher quality have consistently higher-intensity displays than those of lower quality. This honesty may be maintained by a handicap mechanism that ensures that the fitness of higher-quality individuals increases more with additional investment in the display, because they experience smaller marginal viability costs and/or obtain greater marginal fecundity benefits than lower-quality individuals (Getty 1998, 2006; van Doorn and Weissing 2006). Similarly, in Fisherian models of sexual selection, a preference for ornamentation is evolutionarily stable only when display expression directly reflects underlying genetic differences, maintained through biased mutation or other processes (Pomiankowski et al. 1991). In contrast, when display intensity is dynamic or flexible, any given display may not reflect signaler quality. In particular, with a flexible display, variation in display intensity could conceivably undermine signal honesty if individuals of lower quality secure a greater mating advantage than those of higher quality by investing heavily in the display under some conditions and little under others.
No existing model of which we are aware addresses this possibility. Hutchinson et al. (1993) constructed a model of song displays in which a female preference led males to vary the timing of their singing throughout the day. However, the model assumed a fixed female preference for more intense displays. Thus, the model does not shed light on how a preference for a dynamic display trait might arise nor on the patterns of signaling that would be produced as the preference and display coevolve. Similarly, South et al. (2012) showed that a male bias towards courting higher-fecundity females can evolve when females prefer males who invest more in courtship, demonstrating another route to the evolution of signal flexibility. But once again, the female preference was assumed by the modelers, not free to evolve, eliminating the coevolutionary dynamics of present interest.
To fill this theoretical gap, we investigate two intertwined questions: (1) can a flexible, dynamic display coevolve with a sexual preference for that display through a handicap mechanism? (2) If so, how should individuals tactically adjust their investment in the display in response to competition from rival signalers?
We begin by giving sharp definitions to static, dynamic, and flexible displays. We then construct an individual-based simulation of the coevolution of a male static display trait with a female sexual preference, following the standard assumptions of sexual selection theory. Specifically, the model relies on a revealing-handicap mechanism (Maynard Smith 1985), which is well-studied in the case of static displays (van Doorn and Weissing 2006). Under this handicap, for the same investment in the display, males of higher and lower quality pay the same costs, but the former are more attractive to females that have a preference for the display. Note that this condition is different from that for an “index” signal, where display intensity is strictly determined by physiological, anatomical, or other constraints (Bradbury and Vehrencamp 2011); under the revealing handicap and other handicap mechanisms, the expressed display intensity is a strategic choice. We employ a simulation model rather than an analytical one for the greater level of mechanistic detail that simulations allow in specifying the costs and benefits of displays (Kuijper et al. 2012), and for the ease with which they can capture the coevolutionary dynamics between displays and preferences.
We then extend this established model by implementing a display that is dynamic and flexible, rather than static, and a corresponding preference. The display is dynamic in that males express it (and pay its associated cost) only when they actively court a female. The display is flexible in that males may adjust their display investment in response to rival males that they display alongside (who are competing to mate with the same female). There are two reasons that flexibility based on the number of rivals is a compelling avenue for research. First, there is existing empirical evidence of this form of flexibility, for example in fiddler crabs, Austruca mjoebergi, which wave their major claw faster as the level of competition increases (Milner et al. 2012). Second, it is not obvious through intuition alone whether males should, in general, display more or less when they display alongside more rivals. When rival males engage in costly fighting and those costs are sufficiently high, we would expect males to signal less when they face formidable rivals. For example, lekking sage grouse, Centrocercus urophasianus, delay signaling to avoid aggression from rivals (Patricelli et al. 2016). But what about when competition between males acts only through female choice, not direct contests? On the one hand, when a male faces more rivals, he might have to signal more intensely to capture a female’s attention and so obtain a reasonable chance of being chosen to mate. On the other hand, if competition is too intense, it might be better to reduce display effort, or stop displaying altogether. The trade-off between these pressures may favor maximum display intensity at some intermediate number of rivals, though where exactly that maximum falls will depend on the details of the situation. It is precisely this difficulty of giving a satisfying narrative account of the evolutionary outcome that necessitates formal modeling (Otto and Rosales 2020).
Static, dynamic, and flexible displays
We distinguish displays along two lines: first, displays may be either static or dynamic, and secondly, dynamic displays may be either flexible or non-flexible. These dichotomies represent theoretical extremes useful for mapping the subtler variation in empirically observed displays.
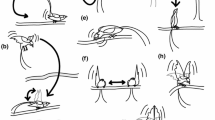
We define static displays as display traits whose intensity is constant on long timescales, normally throughout an individual’s adult lifetime or throughout stages of growth (Fig. 1a). An important property of purely static displays is that they do not require us to distinguish between when an individual is actively displaying and when it is being passively observed, such that the associated costs are independent of how often it encounters potential mates. We can then reasonably model a display as a constant trait value determined in advance of any encounters with potential mates. Biologically, a purely static display can be thought of as a morphological trait that is displayed similarly at all times. For example, the length of a bird’s tail feathers may be considered largely static in the sense that it is always visible and has associated costs (e.g., a reduction in flight performance) that are paid even when the individual is not actively courting.
The costs of three types of display. The blue dotted lines show the survival cost of a hypothetical display at each time step during an individual’s adult lifetime for each type of display, while the yellow lines show the cumulative lifetime cost. The static display’s intensity is constant across time, so the cumulative cost (and benefit, not shown) increases linearly (left). Both the non-flexible, dynamic (center) and flexible, dynamic (right) displays’ intensities, and hence the costs (and benefits, not shown), are zero except at four time steps when the individual actively displays. However, for the non-flexible display, the cost is the same for each display event, whereas for the flexible display, the cost for each display event differs, because the display intensity varies across display contexts. Note that until the first display event, the cost of the dynamic displays is zero
We define dynamic displays as display traits whose intensity is negligible except when the signaler actively displays. In contrast to those of a static display, the cost and benefit of a dynamic display depend on the display’s use; with a purely dynamic display, a signaler that never has the opportunity to display pays no survival cost and gains no mating benefit, regardless of what its investment in the display would have been. For example, birdsong is partially dynamic in that the bird pays part of the cost of displaying, such as increased attention from predators, only while singing and can stop singing to cease paying that cost while also ceasing to receive the benefit.
Following Wainwright et al. (2008), we define flexible displays as display traits whose intensity, and consequently their cost and benefit, varies across different signaling contexts. Such variation is common empirically. For example, male house flies, Musca domestica, adjust their displays according to the differing preferences of potential mates (Meffert and Regan 2002); male Australian terrestrial toadlets, Pseudophryne bibronii, increase their call rate in the presence of a female odor, when the cost-effectiveness of displaying is higher (Byrne and Keogh 2007); male black widows, Latrodectus hesperus, selectively display when the risk of sexual cannibalism is lower (Baruffaldi and Andrade 2015); and male fiddler crabs adjust the rate they wave their claws for females based on the number of rival males waving concurrently (Milner et al. 2012).
Flexible displays are a subset of dynamic displays: because context can alter signal intensity on short timescales, flexible displays are inherently dynamic. As such, there are three possible types of display in our typology: (1) static displays; (2) non-flexible, dynamic displays; and (3) flexible, dynamic displays (Fig. 1). However, few real-world displays are likely to be well approximated as purely static or dynamic or as purely flexible or non-flexible (Patricelli et al. 2016); this typology is merely a theoretical simplification to make models more tractable. In reality, displays may consist of multiple, interacting components, each with varying degrees of dynamism and flexibility depending on the timescale over which they are adjusted, from very rapid movements during a single courtship event to seasonal or even longer-term changes. For example, birdsong is deployed dynamically but may involve up-front investments in the associated cognitive and physiological apparatus (Farrell et al. 2015). Conversely, plumage may be visible at all times, but during active courtship its reflectance may become more salient to both potential mates and predators. Thus, explaining most of the diversity of animal courtship displays requires accounting for dynamism and flexibility.
Model
We model a population of N individuals with non-overlapping, discrete generations and an even primary sex ratio. Each individual has autosomal, diploid genetic values for (1) quality, (2) investment in a static display, (3) investment in a dynamic display, (4) sexual preference for the static display, and (5) sexual preference for the dynamic display. These traits are summarized in Table 1 and take real-number values, following a continuum-of-alleles model (Bürger 1988). Quality is expressed in all individuals, while display expression is limited to males and preference expression is limited to females.
Traits
Quality
Quality, q, incorporates all genetic factors other than display and preference expression that affect viability. q may assume any real value, with q = 0 corresponding to optimum genetic viability. We assume that genetic viability, v, declines exponentially with q according to v = exp( −|q|).
Static display
Males make a one-time investment in their static display during development that reduces their survival to maturity and determines their static display investment, ts, as an adult. For example, ts could represent the size of a morphological ornament that is fixed at the end of development. For ease of biological interpretation, ts is restricted to non-negative values, with a value of 0 corresponding to no ornament and larger values corresponding to larger ornaments.
Dynamic display
The dynamic display function, td(n), gives a male’s investment in the dynamic display when displaying alongside n rival males. We assume that during a courtship encounter a female always mates with one of the displaying males. As a consequence, it is optimal for a male to invest nothing in a display when he has no rivals, because he is guaranteed to mate regardless. Thus, we allow a male’s dynamic display investment when displaying alone, d0, to evolve separately from his investment when displaying alongside rivals. Because there is little empirical evidence on the functional form that td might take in nature, we assume for simplicity that td is linear in n for n > 0. Finally, we constrain the display investment to non-negative values, yielding
Intuitively, a male invests a baseline effort of d1 when displaying alongside the mean number of rivals, \(\overline{n}\), and adjusts this investment upwards, downwards, or not at all according to n and the sign of d2. If d2 > 0, the male displays more alongside more rivals; if d2 < 0, he displays less. When d2 = 0 and d0 = d1, the display is dynamic (it is only expressed during courtship events) but non-flexible (investment in it is always the same, including when displaying alone), whereas when d2 ≠ 0 and/or d0 ≠ d1, the display is both dynamic and flexible (investment in it varies between display events).
Preferences
For simplicity, we assume that female preferences are fixed across their lifetime. Let ps be a female’s preference for the static display and pd her preference for the dynamic display. A positive value of ps or pd implies a preference for males with higher display intensity, while a negative value implies a preference for males with lower display intensity. A value of zero implies no preference, i.e., random mating.
Procedure
Each generation, we simulate three processes in sequence: mortality, nonrandom mating, and reproduction. Figure 2 illustrates the simulation procedure for each generation.
Mortality
At the start of the generation, each individual survives according to its initial viability, as determined by its genotype. Female viability, Sf, increases with genetic viability v and decreases with preferences ps and pd according to
where the cost parameter α determines how quickly viability decreases with increasing preference. Similarly, male viability, Sm, increases with genetic viability v and decreases with static display expression ts according to
where the cost parameter βs determines how quickly a male’s viability decreases with investment in the static display. This expression for male viability accounts for survival to adulthood, before any courtship. In contrast to the static display cost, the dynamic display cost has no effect on this initial viability. Instead, the dynamic display cost reduces viability after each display, as described below.
Nonrandom mating
Next, we randomly draw one female N times, with replacement, from the surviving population. In sequence, each female drawn randomly samples n + 1 surviving males, where n is a Poisson-distributed random number with a mean of \(\overline n\). (Note that we add the 1 to this number to ignore cases where a female samples zero males). Thus, \(\overline n\) is the mean number of rival males sampled, that is, the number of males in addition to any focal male in a sample.
Each of the sampled males then displays to the female, with an investment of ts in the static display and td(n) in the dynamic display. We constrain the investment in each display to non-negative values for ease of interpretation.
A male’s attractiveness, A, as perceived by a female with preferences ps and pd, is given by
Note that attractiveness increases more with display investment for males of higher viability. The model thus implements a revealing handicap, following the implementation of van Doorn and Weissing (2006). The biological interpretation is that, for the same level of investment in the display, the realized display intensity is greater for males of higher quality. Consequently, higher-quality males will obtain greater marginal fitness returns than lower-quality males for a given increase in display investment, which is the key requirement of the handicap principle (Getty 2006). For example, if a male is otherwise more vigorous, then the same investment in a motor display might correspond to a more skillful display. The choosing female then selects and mates with one of the displaying males with a probability proportional to A.
After displaying and possibly being selected by the female to mate, each male survives with probability \(\exp\left(-\beta_{\mathit d}t_{\mathit d}^{\mathit2}\right)\), where the cost parameter βd determines how quickly survival decreases with dynamic display investment in the current courtship attempt. Biologically, this survival risk could represent the display attracting the attention of predators. Thus, by investing in the dynamic display, a male increases his probability of being chosen by the current female (assuming pd > 0 for that female) but reduces his probability of surviving to be sampled by future females.
Reproduction
Each mating produces one offspring. We determine the genetic values of the offspring through standard Mendelian inheritance, assuming no genetic dominance and that all loci are unlinked.
Following established models of sexually selected handicaps (e.g., Iwasa et al. 1991; van Doorn and Weissing 2006; Henshaw et al. 2022), we assume that in each offspring the preference and display traits each mutates with probability μt, quality mutates with probability μq, and mutations in displays and preferences are unbiased, while mutations in quality are downward-biased (i.e., q is more likely to decrease than increase). If a mutation occurs in a display or preference trait, we simulate the mutation by adding a normal deviate with a mean of zero and a small standard deviation, σt, to the genetic value. If a mutation occurs in quality, we first compute the magnitude of the mutation as the absolute value of a normal deviate of mean zero with standard deviation σq, then we determine the sign of the change: the mutation is downward (negative) with probability γ, where γ > 0.5, and upward with probability 1 − γ.
Simulations
To separate the effects of dynamism and flexibility in display expression, we run three sets of simulations. In the first set, we analyze the results of the baseline static-display model. For these runs, we set the static display cost βs to two values, 0.15 and 0.2, and run 40 replicates for each value. We initialize the preference for the static display ps at 1 and static display investment ts at 0.
In the second set of simulations, we replace the static display with a dynamic display but require that males invest the same effort in every display they give (i.e., the display is dynamic but non-flexible, with d0 = d1 and d2 = 0). We set the dynamic display cost βd to two values, 0.032 and 0.035, and again run 40 replicates for each value. We initialize the preference for the dynamic display pd at 1 and dynamic display investment d0 = d1 at 0.
In the third set of simulations, we incorporate flexibility by allowing males to vary their investment in the dynamic display in response to the number of rivals they face. We set the dynamic display cost, βd, to two values, 0.032 and 0.035, and once again run 40 replicates for each value. We initialize the preference for the dynamic display, pd, at 1 and dynamic display investment d0, d1, and d2 at 0.
For all three sets of simulations, we set the values of all other parameters as in Table 1. All simulations span 40,000 generations of evolution. To reveal the effects of selection more clearly, in all simulations, we assume a pre-existing female preference for the display, holding the preference constant at its initial value for the first 5000 generations while allowing the male display to evolve. Then, once evolutionary change in the display investment has slowed down, we allow the female preference to coevolve with the male display. Similarly, to isolate the effects of display flexibility in the simulations that include the flexible, dynamic display, we constrain d0 = d1 and d2 = 0 for the first 10,000 generations, while allowing the baseline display investment, d1, to evolve, before allowing the three display components to coevolve with one another and with the female preference.
Results
Static display
The static display and preference coevolved to positive stable values (Fig. 3a), in line with the results of established sexual selection models (Kuijper et al. 2012). Also as expected, once the two traits neared these values, we observed a positive relationship between the two traits: the display evolved to higher values when the preference was high (Fig. 3b).
The static display and preference coevolve to positive, stable values. a Evolution of the static display (blue, D) and preference for that display (yellow, P). The thin lines show the evolutionary trajectories for 10 individual replicates while the thick lines show the mean value across all replicates. The dotted line marks generation 5000, after which the preference was allowed to mutate. b Static display versus the preference for that display. The thick line shows the trajectory for the replicate with the median final display value while the thin lines show the trajectories for all other replicates. The vertical dotted line marks zero display intensity. c Display intensity versus natural log genetic viability (ln v = −|q|) for displaying males in the final generation for the replicate with the median final correlation between those two traits. Each data point corresponds to a single display event and shows the associated display intensity and male quality. The dashed line shows the fitted regression line for the two variables. d Distribution of correlation coefficients (Pearson’s r) for display intensity versus natural log genetic viability for displaying males in the final generation, for two different display costs. Each data point shows the correlation coefficient for one replicate; boxplots show the median, interquartile range, and whiskers (extending to 1.5 times the interquartile range). The horizontal dotted line marks r2 = 0. Static display cost βs = 0.2 in a, b, and c, and all other parameter values are as in Table 1
For the static display to function as an honest indicator of male quality, there must be a positive relationship between displaying males’ genetic viability and their realized display intensity. To assess this relationship, for each replicate we calculated the r2 for the realized display intensity of every display given in the final generation versus the log viability (ln v = −|q|) of the displaying male (note that males displaying more than once are counted multiple times in this relationship). The r2 thus measures how well male quality is predicted by the variation in the display intensity that females observe.
In the final generation we observed (Fig. 3c, d) a positive display-viability relationship (mean r2 = 0.66, 95% CI [0.63, 0.69], across replicates with βs = 0.2). We can conclude that static display intensity evolves to be an honest indicator of quality, supporting the evolution of a costly preference for the display, in line with the findings of established models of sexual selection (Kuijper et al. 2012).
Non-flexible, dynamic display
As with the static display, the non-flexible, dynamic display and the preference for it evolved to stable, positive values (Fig. 4a), with a positive relationship between the two traits (Fig. 4b).
The non-flexible, dynamic display and preference coevolve to positive, stable values, similar to the static display. All data are analogous to those in Fig. 3, but for the non-flexible, dynamic display instead of the static display. Dynamic display cost βd = 0.035 in (a), (b), and (c), and all other parameter values are as in Table 1
Note that it is not informative to directly compare the equilibrium values for static and non-flexible, dynamic display intensities. This is because those values depend on the costs of the respective displays, which differ in two key respects. First, on average, males display multiple times and so pay the cost of a dynamic display more than once (hence, we have set the per-display dynamic cost, βd, an order of magnitude below the lifetime static cost, βs). Second, males must display at least once before incurring any cost of the dynamic display. Thus, for males that display once or not at all, the dynamic display is no more costly when βd is low than when βd is high. For these two reasons, we should not expect a priori the values for the static and dynamic displays to evolve to similar levels when the parameters βs and βd are similar. However, it is possible to directly compare the equilibrium values for the non-flexible and flexible dynamic displays, as they depend on the same cost parameter, implemented identically.
As with the fixed display, we observed a positive relationship (mean r2 = 0.35, 95% CI [0.33, 0.37], across replicates with βd = 0.035) between displaying males’ genetic viability and their display intensity in the final generation (Fig. 4c, d), showing that non-flexible, dynamic display intensity can evolve to be an honest indicator of quality.
These findings are novel but not altogether surprising. They are novel in that no previous model of which we are aware has demonstrated the coevolution of a non-flexible, dynamic display with a sexual preference. Our results show that a non-flexible, dynamic display can evolve to an exaggerated level under a revealing handicap mechanism. The findings are unsurprising because, just as with a static display, each male has a single level of display intensity. Thus, there is no way for lower-quality males to undermine signal honesty by displaying more intensely under favorable conditions. As such, we should expect a revealing handicap to function with a non-flexible, dynamic display similarly to how it does with a static display: males of lower-quality will consistently display less intensely than those of higher quality.
Flexible display
As with the static and non-flexible, dynamic displays, the flexible display and preference for it coevolved to stable, positive values (Fig. 5a), with a positive relationship between the two traits (Fig. 5b). In the final generation, we observed (Fig. 5c, d) a positive correlation between displaying males’ genetic quality and display intensity (mean r2 = 0.078, 95% CI [0.075, 0.081], across replicates with βd = 0.035). It follows that flexible display intensity evolved to be an honest indicator of quality. However, the r2 value is considerably lower than that for the non-flexible static display, meaning that the flexible, dynamic display is a much noisier indicator of male quality than the non-flexible, dynamic display. The likely source of this noise is the evolved strategy of adjusting display investment in response to random variation in the number of competitors.
The flexible, dynamic display and preference coevolve to positive, stable values, similar to the static and non-flexible, dynamic displays, but the display is a weaker signal of male quality. All data are analogous to those in Fig. 3, but for the flexible display instead of the static display. In a, the blue lines show males’ baseline investment in the flexible display, d1, that occurs when displaying alongside the average number of rival males, \(\overline n\) The vertical dotted lines mark generation 5000, after which the preference and was allowed to mutate from its initial value, and generation 10,000, after which the display investment when displaying alone, d0, was allowed to mutate away from d1 and the display slope, d2, was allowed to mutate away from zero. Dynamic display cost βd = 0.035 in a, b, and c, and all other parameter values are as in Table 1
Intriguingly, despite the flexible display being a noisier indicator of quality, the preference for the flexible display did not evolve to a significantly lower value than that for the non-flexible, dynamic display by the final generation, even as the baseline investment in the flexible display, d1, evolved to a higher value for the flexible display than for the non-flexible, dynamic display (0.09 points higher, 95% CI [0.03, 0.15], across replicates with βd = 0.035). Note that we cannot draw a similar comparison with the r2 values or the equilibrium display investment for static display because those values partly depend on the non-comparable display cost parameters βd and βs (e.g., see Fig. 3d).
The key way that display flexibility might undermine signal honesty is by allowing lower-quality males to invest more heavily in the display in particular signaling contexts such that their displays are more intense than those of higher-quality males. However, this route to signal dishonesty would require that sufficient genetic covariance accumulate between quality and the genetically determined display investment strategy. In contrast, in the present model, the relationship between quality and realized display intensity approximated the fixed relationship dictated by the revealing handicap, namely that the display intensity increases exponentially in v, as shown in Fig. 5c. We further find no relationship between the quality and the dynamic display slope, d2, of displaying males in the final generation, indicating that there is no systematic difference in display flexibility between low-viability and high-viability males. We can conclude that (a) flexibility does not inherently favor males of lower (or higher) quality and/or (b) whatever advantage flexibility might provide is obscured by recombination preventing the buildup of genetic covariance between quality and display investment.
These results answer the first of our initial questions: a flexible display, where display investment varies with the number of rival males, can evolve under a revealing handicap. Turning to our second question, we now examine the patterns of display flexibility that resulted from the coevolutionary process. As expected, males evolve to invest nothing in the display when displaying alone. The genetic value for display investment when displaying alone, d0, falls to zero and then drifts downwards to negative values (Fig. 6a), as positive mutations are selected against, while the realized display intensity is constrained to non-negative values, resulting in an overall display investment of zero. Meanwhile, the display slope, d2, rapidly evolves to a negative equilibrium value (Fig. 6c) and remains there for the rest of the simulation (mean − 0.20, 95% CI [− 0.21, − 0.19], across replicates with βd = 0.035 in the final generation). Figure 6d depicts the final display function for each replicate. These results suggest that, in the scenario we have modelled, when males display alongside at least one rival, they should reduce their display intensity as the number of rivals increases.
Males evolve to display less intensely when they face more rivals and not display at all when they display alone. a Evolution of dynamic display investment when displaying alone, d0. The horizontal dotted line corresponds to zero display investment when the number of rivals n = 0. Note that negative values result in a display intensity of zero. b Evolution of baseline dynamic display investment, d1. The horizontal dotted line corresponds to zero display investment when displaying alongside the mean number of rivals, \(\overline{n}\). The data in this panel are the same as those illustrated in blue in Fig. 5a. c Evolution of dynamic display slope, d2. The horizontal dotted line corresponds to display investment that is constant with respect to the number of rival males when there is at least one rival (d2 = 0 for n > 0). d Dynamic display investment td (n) versus number of rival males n in the final generation. The thin lines show the display functions for all 40 replicates based on mean values of d0, d1, and d2 in the final generation. The thick line shows the display function where d0, d1, and d2 are the mean across all replicates for the final generation. In a, b, and c, the thin blue lines show the trajectories for 10 individual replicates, while the thick blue line shows the mean values across all 40 replicates. The vertical dotted lines mark generation 5000, after which the preference and was allowed to mutate from its initial value, and generation 10,000, after which the display investment when displaying alone, d0, was allowed to mutate away from d1 and the display slope, d2, was allowed to mutate away from zero. Dynamic display cost βd = 0.035, and all other parameter values are as in Table 1
Discussion
The model presented here is the first to investigate how a sexual preference and a flexible, dynamic courtship display coevolve. We began with an established model of sexual selection (the static-display model) and reproduced the established result that a display trait and sexual preference can coevolve to exaggerated levels under a revealing handicap mechanism (Iwasa et al. 1991; van Doorn and Weissing 2006). But this version of the model, and the prior models that inspired it, only closely resemble a narrow class of empirically observed courtship displays: those whose expression (and costs) are fixed across an individual’s lifetime or stages of growth. In reality, even morphological ornaments (e.g., plumage) are often just one component of more complex, dynamic displays (e.g., a dance) that may alter the ornaments’ perceived intensity. To account for this much broader class of displays whose expression varies between courtship events, we extended the established model to include dynamic and flexible display traits. Our results showed that dynamic displays can coevolve with a sexual preference to exaggerated levels through the same handicap mechanism as a static display. When males were allowed to flexibly adjust their display investment based on the number of rivals courting the same female, the display became a far noisier indicator of male quality, but the costly female preference still evolved and still supported costly display expression.
The extended model also revealed how males evolved to tactically adjust their display investment. In the scenario we modelled, males evolved to express the minimum display intensity when displaying alone and showed the highest display intensity when competing against exactly one rival signaler, decreasing their display investment when additional rivals were present. This result is analogous to predictions from models of sperm competition intensity, where in the presence of competing ejaculates, the optimal investment in each mating event decreases as the number of competing ejaculates increases (Parker and Ball 2005).
The central significance of our work is that it shows that some key results and insights of established models of static displays generalize to a much larger class of courtship displays. These results, however, should be taken as preliminary—a first step towards generalizing sexual selection theory to account for dynamism and flexibility in display expression. Numerous extensions to the present model that could enhance its biological realism immediately suggest themselves. We restrict ourselves to considering seven here.
First, the linear function is just one simple functional form for the flexible display. A key advantage of the linear display function is that it is straightforward to analyze, both because it takes only two parameters (plus one for the case of no rivals) and because linearity ensures that a mutation of any given magnitude alters the overall display investment by a fixed amount. (If, for example, the display function were instead accelerating in any of its parameters, then the effect of mutations would be greater when the existing investment in the display was high.) The trade-off for this simplicity is reduced flexibility. In the present model, males’ investment can neither accelerate nor decelerate in the number of rivals. To determine the model’s robustness to this restriction, future research could add a curvature parameter, d3, to the display function (e.g., \(d_{1}+d_{2}n^{d_{3}}\) rather than d1 + d2n) or investigate alternate forms, such as polynomials with additional terms, a logistic function, or separate investment levels for each number of rivals, restricting that number to a finite set of values. Ultimately, empirical evidence is required to determine a realistic functional form. For example, male fiddler crabs appear to adjust their waving rate little between one and two waving neighbors and then increase their waving rate approximately linearly with higher numbers of rivals (Milner et al. 2012), which is a very different pattern from that predicted by our model. Further theoretical work is needed to understand how the pattern of display adjustment should vary depending on the biological details of mate choice, including female sampling strategies and the degree of flexibility in male courtship. For example, “best-of-n” sampling by females might favor a qualitatively different pattern of competitive courtship from “threshold” sampling (Wiegmann et al. 2013), because the successful male is the one who outshines his rivals, rather than the first one to surpass the female’s pre-existing threshold. Furthermore, if males are directly sensitive to the display intensity of their rivals (which they were not in our model) and can respond by upping their own courtship intensity, we might expect display investment to increase, rather than decrease, with greater numbers of rivals. Extensions of our model are needed to understand these issues better.
Second, we have assumed that the same genes determine display expression in males of differing qualities. If instead the genes that determine quality also determined which genes coding for display were expressed, then it would be possible for low-quality display investment and high-quality display investment to evolve as separate traits. This would allow signaling strategies that deviate more from the fixed relationship dictated by the revealing handicap. For example, low-quality display investment could evolve to a higher level than high-quality display expression, partially or fully counteracting the effects of the revealing handicap. Modeling this scenario would allow a more thorough investigation of the conditions under which the revealing handicap supports signal honesty (van Doorn and Weissing 2006) for static, dynamic, and flexible displays.
Third, we have assumed that females do not adjust preference strength by the number of rival males. Yet if males can perceive the number of rivals they display alongside and respond accordingly, it seems plausible to assume that females could use the same information. If females adjusted the strength of their preferences to counteract males’ increased (or reduced) display effort, this could potentially reduce the fitness advantage of displaying flexibly. In future work, a flexible preference could be modeled in a directly analogous way to the flexible display (e.g., \(p(n) = {p}_{1} +(n -\overline{n}) {p}_{2}\) for n > 0). It would then be possible to the investigate all combinations of flexible and non-flexible preferences and static, non-flexible dynamic, and flexible dynamic displays. In such a model, a flexible preference could plausibly evolve for a static display, for example a preference for long tail feathers that grows stronger when more potential mates are available to choose between. To our knowledge, the coevolutionary dynamics of such a scenario have yet to be explored.
Fourth, we have modeled the display as either purely static or purely dynamic, but most displays in nature have both static and dynamic components (Patricelli et al. 2016). Past models suggest that it is difficult to sustain costly preferences for multiple indicators of the same aspect of quality (van Doorn and Weissing 2004), so given the dynamic display’s poorer information content, we might expect the dynamic display to be lost and the static display retained if the two were initially active in the present model as separate, non-interacting traits. However, the two displays could plausibly synergize with one another as part of a more complex, overall display, such as when a dance shows off bright plumage. The model could be extended to account for this scenario by including both the static and the dynamic display, td and ts; the female preferences for both displays, ps and pd; and a preference px for the interaction between the static and dynamic components (i.e., ts × td). Then, the display could evolve to be completely static (td = 0), completely dynamic (ts = 0), or in between (ts ≠ 0, td ≠ 0), with females potentially preferring a level of dynamism anywhere along that spectrum.
Fifth, we have assumed that the flexible display imposes a cost only through decreased viability. However, when multiple displays take place within a short time frame, investing in the display might also reduce the signaler’s condition, impairing further displays for a limited time. Changes in current condition would then introduce extra variation in the display intensity of a male of any given quality. Then, we might expect condition-dependent signaling strategies to evolve, such that males might compensate for their fatigue by increasing their investment or conserve their energy by decreasing their investment. A state-dependent model would be needed to analyze such strategies and would differ fundamentally from the viability-based model developed here, in that the fatigue induced by the dynamic display (whether non-flexible or flexible) could wear off over time, and hence the cost of the display would depend crucially on investment in recent display events.
Sixth, the present model does not incorporate signal interference or male-male competition, both of which may affect the evolution of flexible display strategies. For example, male bird-voiced tree frogs, Hyla avivoca, modify their calls to avoid overlap with the calls of rivals (Martínez-Rivera and Gerhardt 2008), indicating that acoustic interference may be an important driver of display flexibility. As another example, male sage grouse have been observed to avoid signaling when doing so would risk a fight with rival males present at a lek (Patricelli et al. 2016), suggesting contests and injury as another potential driver of display flexibility.
Seventh, we have allowed males to adjust their display investment based only on the number of rivals displaying. But males might plausibly adjust their displays based on a variety of other cues. For example, when females vary in quality or fecundity, males might alter their investment based on cues to those traits, increasing their courtship effort for more desirable mates. Alternatively, if displaying attracts predators, males might plausibly display more intensely when and where the risk of predation appears lower. Another possibility is that, when the population density varies over time, males might adjust their display intensity based on the frequency of encounters with potential mates. Yet, another possibility is that males might display more intensely when alternative activities, such as food gathering, are less worthwhile, such as in poor light conditions. For example, such variations in the opportunity cost of displaying may explain why birdsong often peaks at twilight (Hutchinson et al. 1993). To our knowledge, no existing model of the coevolution of a display and preference has incorporated any of these sorts of tactical display adjustment.
We have, in sum, focused entirely on the display flexibility that arises due to female choice between varying numbers of rival males and showed, for the first time, that trait exaggeration can occur with such flexibility. The vast array of other empirically documented and theoretically compelling sources of within-individual display variation outlined above—yet to be integrated into established sexual selection theory—await modeling. After decades of theory have revealed a rich picture of the evolution of static sexual ornaments, it is time to generalize the field’s findings to account for the ubiquitous dynamism of animal courtship.
Code and data availability
The data sets generated during the current study, as well as the simulation code used to generate it, are available in the following repository: https://doi.org/10.5061/dryad.vx0k6djvt.
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Andersson M, Simmons LW (2006) Sexual selection and mate choice. Trends Ecol Evol 21:296–302. https://doi.org/10.1016/j.tree.2006.03.015
Baruffaldi L, Andrade MC (2015) Contact pheromones mediate male preference in black widow spiders: Avoidance of hungry sexual cannibals? Anim Behav 102:25–32. https://doi.org/10.1016/j.anbehav.2015.01.007
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication, 2nd edn. Sinauer, Sunderland
Bürger R (1988) Mutation–selection balance and continuum-of-alleles models. Math Biosci 91:67–83. https://doi.org/10.1016/0025-5564(88)90024-7
Byers J, Hebets E, Podos J (2010) Female mate choice based upon male motor performance. Anim Behav 79:771–778. https://doi.org/10.1016/j.anbehav.2010.01.009
Byrne PG, Keogh JS (2007) Terrestrial toadlets use chemosignals to recognize conspecifics, locate mates and strategically adjust calling behaviour. Anim Behav 74:1155–1162. https://doi.org/10.1016/j.anbehav.2006.10.033
Farrell T, Kriengwatana B, MacDougall-Shackleton SA (2015) Developmental stress and correlated cognitive traits in songbirds. Comp Cogn Behav Rev 10:1–23. https://doi.org/10.3819/ccbr.2015.100001
Getty T (1998) Handicap signalling: When fecundity and viability do not add up. Anim Behav 56:127–130. https://doi.org/10.1006/anbe.1998.0744
Getty T (2006) Sexually selected signals are not similar to sports handicaps. Trends Ecol Evol 21:83–88. https://doi.org/10.1016/j.tree.2005.10.016
Henshaw JM, Fromhage L, Jones AG (2022) The evolution of mating preferences for genetic attractiveness and quality in the presence of sensory bias. P Natl Acad Sci USA119:e2206262119. https://doi.org/10.1073/pnas.2206262119
Hutchinson JMC, McNamara JM, Cuthill IC (1993) Song, sexual selection, starvation and strategic handicaps. Anim Behav 45:1153–1177. https://doi.org/10.1006/anbe.1993.1139
Iwasa Y, Pomiankowski A, Nee S (1991) The evolution of costly mate preferences II. the “handicap” principle. Evolution 45:1431–1442. https://doi.org/10.1111/j.1558-5646.1991.tb02646.x
Kokko H, Brooks R, McNamara JM, Houston AI (2002) The sexual selection continuum. Proc R Soc Lond B 269:1331–1340. https://doi.org/10.1098/rspb.2002.2020
Kuijper B, Pen I, Weissing FJ (2012) A guide to sexual selection theory. Annu Rev Ecol Evol S 43:287–311. https://doi.org/10.1146/annurev-ecolsys-110411-160245
Martínez-Rivera CC, Gerhardt HC (2008) Advertisement-call modification, male competition and female preference in the bird-voiced treefrog Hyla avivoca. Behav Ecol Sociobiol 63:195–208. https://doi.org/10.1007/s00265-008-0650-0
Maynard Smith J (1985) Sexual selection, handicaps and true fitness. J Theor Biol 115:1–8
Mead LS, Arnold SJ (2004) Quantitative genetic models of sexual selection. Trends Ecol Evol 19:264–271. https://doi.org/10.1016/j.tree.2004.03.003
Meffert LM, Regan JL (2002) A test of speciation via sexual selection on female preferences. Anim Behav 64:955–965. https://doi.org/10.1006/anbe.2002.2020
Milner RNC, Jennions MD, Backwell PRY (2012) Keeping up appearances: male fiddler crabs wave faster in a crowd. Biol Lett 8:176–178. https://doi.org/10.1098/rsbl.2011.0926
Otto SP, Rosales A (2020) Theory in service of narratives in evolution and ecology. Am Nat 195:290–299. https://doi.org/10.1086/705991
Parker G, Ball M (2005) Sperm competition, mating rate and the evolution of testis and ejaculate sizes: a population model. Biol Lett 1:235–238. https://doi.org/10.1098/rsbl.2004.0273
Patricelli G, Krakauer A, Taff C (2016) Variable signals in a complex world. Adv Stud Behav 48:319–386. https://doi.org/10.1016/bs.asb.2016.02.002
Pomiankowski A, Iwasa Y, Nee S (1991) The evolution of costly mate preferences I Fisher and biased mutation. Evolution 45:1422–1430. https://doi.org/10.1111/j.1558-5646.1991.tb02645.x
South SH, Arnqvist G, Servedio MR (2012) Female preference for male courtship effort can drive the evolution of male mate choice. Evolution 66:3722–3735. https://doi.org/10.1111/j.1558-5646.2012.01716.x
Spezie G, Quigley C, Fusani L (2022) Learned components of courtship: a focus on postural displays, choreographies and construction abilities. Adv Stud Behav 54:43–108
van Doorn GS, Weissing FJ (2004) The evolution of female preferences for multiple indicators of quality. Am Nat 164:173–186
van Doorn GS, Weissing FJ (2006) Sexual conflict and the evolution of female preferences for indicators of male quality. Am Nat 168:742–757
Wainwright PC, Mehta RS, Higham TE (2008) Stereotypy, flexibility and coordination: key concepts in behavioral functional morphology. J Exp Biol 211:3523–3528. https://doi.org/10.1242/jeb.007187
Wiegmann D, Angeloni L, Seubert S, Wade G (2013) Mate choice decisions by searchers. Curr Zool 59:184–199. https://doi.org/10.1093/czoolo/59.2.184
Acknowledgements
We thank the editor, Jan Lindström, and three anonymous reviewers for their helpful comments.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
TWF and IGR conceived the study and built an initial version of the model. SH developed the full model with input from TWF and TV, analyzed the output, and wrote the first draft of the manuscript. All authors commented on the manuscript and read and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Communicated by J. Lindström
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hollon, S.H., García-Ruiz, I., Veen, T. et al. The evolution of dynamic and flexible courtship displays that reveal individual quality. Behav Ecol Sociobiol 77, 24 (2023). https://doi.org/10.1007/s00265-023-03296-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03296-9