Abstract
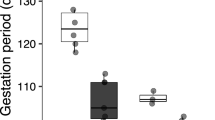
Early developmental temperatures influence the physiology and behavior of reptiles, with important consequences for their fitness and survival. For example, many viviparous lizards are cold adapted which may restrict their activity times during the reproductive season, increasing their susceptibility to global warming. However, it is unclear if and how lizards with different reproductive modes (oviparity vs. viviparity) can respond to rising temperatures by modifying their life-history traits. We examined the effect of developmental temperatures on hatchling behavior and learning in viviparous and oviparous populations of the lizard Saiphos equalis to test whether their reproductive behavior can buffer against rising temperatures. Gravid females from both populations were subjected to current or projected end-of-century (future) thermal environments to evaluate differences in the exploratory, foraging and antipredator behavior, and spatial learning ability of their offspring. We found that viviparous lizards were more exploratory and had a less-pronounced antipredator response than oviparous lizards. Regardless of the mode of reproduction, elevated temperatures reduced the exploratory behavior of hatchling lizards. Elevated temperatures also reduced the foraging efficiency of oviparous, but not viviparous, hatchlings. Finally, future-gestated oviparous hatchlings were more likely to choose the correct refuge and made fewer mistakes in a spatial learning task; however, we found only weak evidence of spatial learning in S. equalis. Our results suggest that although global warming is likely to have a negative impact on phenotypic traits, in S. equalis, some of these effects may be ameliorated by maternal behavior and/or physiological responses during pregnancy, particularly in viviparous populations.
Significance statement
Computational modeling studies suggest that live-bearing lizards (viviparous) are more vulnerable to global warming compared with egg-laying ones (oviparous). However, there is little experimental evidence showing that viviparous species are indeed at a greater risk of extinction. Using a lizard species that has both oviparous and viviparous populations, we tested the effect of high developmental temperatures (projected for 2100) on the behavior and learning of their offspring. We found that elevated temperatures had a stronger negative effect on egg-laying lizards by producing hatchlings with lower foraging efficiency. Our results suggest that viviparous mothers can ameliorate some of the effects of global warming on their offspring. Moreover, our study suggests that if live bearers are indeed more vulnerable to global warming, it is likely not due to maladaptive behavior in offspring, but rather, to other causes that affect pregnant females.




Similar content being viewed by others
References
Abayarathna T, Webb JK (2020) Effects of incubation temperatures on learning abilities of hatchling velvet geckos. Anim Cogn (published online). https://doi.org/10.1007/s10071-020-01365-4
Adolph SC, Porter WP (1993) Temperature, activity, and lizard life histories. Am Nat 142:273–295
Amiel JJ, Lindström T, Shine R (2014) Egg incubation effects generate positive correlations between size, speed and learning ability in young lizards. Anim Cogn 17:337–347
Amiel JJ, Shine R (2012) Hotter nests produce smarter young lizards. Biol Lett 8:372–374
Angilletta MJ, Montgomery LG, Werner YL (1999) Temperature preference in geckos: diel variation in juveniles and adults. Herpetologica 55:212–222
Bajer K, Horváth G, Molnár O, Török J, Garamszegi LZ, Herczeg G (2015) European green lizard (Lacerta viridis) personalities: linking behavioural types to ecologically relevant traits at different ontogenetic stages. Behav Process 111:67–74
Ballen CJ, Shine R, Olsson M (2015) Developmental plasticity in an unusual animal: the effects of incubation temperature on behavior in chameleons. Behaviour 152:1307–1324
Bartoń K (2018) MuMIn: Multi-model inference. R package version 1.42.1. Available from https://cran.r-project.org/web/packages/MuMIn/index.html
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Beuchat CA (1986) Reproductive influences on the thermoregulatory behavior of a live-bearing lizard. Copeia 1986:971–979
Beuchat CA (1988) Temperature effects during gestation in a viviparous lizard. J Therm Biol 13:135–142
Birchard GF, Marcellini D (1996) Incubation time in reptilian eggs. J Zool 240:621–635
Biro PA, Abrahams MV, Post JR, Parkinson EA (2004) Predators select against high growth rates and risk–taking behaviour in domestic trout populations. Proc R Soc Lond B 271:2233–2237
Blackburn DG (2006) Squamate reptiles as model organisms for the evolution of viviparity. Herpetol Monogr 20:131–146
Booth DT (2000) Incubation of eggs of the Australian broad-shelled turtle, Chelodina expansa (Testudinata: Chelidae), at different temperatures: effects on pattern of oxygen consumption and hatchling morphology. Aust J Zool 48:369–378
Brattstrom BH (1979) Amphibian temperature regulation studies in the field and laboratory. Integr Comp Biol 19:345–356
Burger J (1989) Incubation temperature has long-term effects on behaviour of young pine snakes (Pituophis melanoleucus). Behav Ecol Sociobiol 24:201–207
Burger J (1991) Effects of incubation temperature on behavior of hatchling pine snakes: implications for reptilian distribution. Behav Ecol Sociobiol 28:297–303
Burger J (1998) Antipredator behaviour of hatchling snakes: effects of incubation temperature and simulated predators. Anim Behav 56:547–553
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: A practical information-theoretic approach, 2nd edn. Springer Verlag, New York, pp 98–143
Bustard HR (1964) Reproduction in the Australian rain forest skinks, Saiphos equalis and Sphenomorphus tryoni. Copeia 1964:715–716
Chamaillé-Jammes S, Massot M, Aragón P, Clobert J (2006) Global warming and positive fitness response in mountain populations of common lizards Lacerta vivipara. Glob Chang Biol 12:392–402
Clark BF, Amiel JJ, Shine R, Noble DWA, Whiting MJ (2014) Colour discrimination and associative learning in hatchling lizards incubated at “hot” and “cold” temperatures. Behav Ecol Sociobiol 68:239–247
Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A (2010) Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc R Soc Lond B 277:1571–1579
Cox RM, Duryea MC, Najarro M, Calsbeek R (2011) Paternal condition drives progeny sex-ratio bias in a lizard that lacks parental care. Evolution 65:220–230
Day LB, Crews D, Wilczynski W (1999) Spatial and reversal learning in congeneric lizards with different foraging strategies. Anim Behav 57:393–407
Dayananda B, Webb JK (2017) Incubation under climate warming affects learning ability and survival in hatchling lizards. Biol Lett 13:20170002
Deeming DC (2004) Post-hatching phenotypic effects of incubation in reptiles. In: Deeming DC (ed) Reptilian incubation environment, evolution and behaviour. Nottingham University Press, Nottingham, pp 229–252
Deeming DC, Ferguson MJW (1991) Physiological effects of incubation temperature on embryonic development in reptiles and birds. In: Deeming DC, Ferguson MWJ (eds) Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, pp 147–171
Dingemanse NJ, Both C, Drent PJ, van Oers K, van Noordwijk AJ (2002) Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim Behav 64:929–938
Dowdy A, Abbs D, Bhend J et al (2015) East coast cluster report. In: Ekström M, Whetton P, Gerbing C, Grose G, Webb L, Risbey J (eds) Climate change in Australia projections for Australia’s natural resource management regions: cluster reports. CSIRO and Bureau of Meteorology, Melbourne, pp 1–53
Downes SJ (2001) Trading heat and food for safety: costs of predator avoidance in a lizard. Ecology 82:2870–2881
Downes SJ, Shine R (1999) Do incubation-induced changes in a lizard’s phenotype influence its vulnerability to predators? Oecologia 120:9–18
Elphick MJ, Shine R (1998) Long-term effects of incubation temperatures on the morphology and locomotor performance of hatchling lizards Bassiana duperreyi (Scincidae). Biol J Linn Soc 63:429–447
Fairbairn J, Shine R, Moritz C, Frommer M (1998) Phylogenetic relationships between oviparous and viviparous populations of an Australian lizard (Lerista bougainvillii, Scincidae). Mol Phylogenet Evol 10:95–103
Foster CSP, Thompson MB, Dyke JUV, Brandley MC, Whittington CM (2020) Emergence of an evolutionary innovation: gene expression differences associated with the transition between oviparity and viviparity. Mol Ecol (published online). https://doi.org/10.1111/mec.15409
Fox CW, Mousseau TA (1998) Maternal effects as adaptations for transgenerational phenotypic plasticity in insects. In: Mousseau TA, Fox CW (eds) Maternal effects as adaptations. Oxford University Press, New York, pp 159–177
Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21:394–407
Gray JE (1825) A synopsis of the genera of reptiles and Amphibia, with a description of some new species. Ann Philos 10:193–217
Hallmann K, Griebeler EM (2015) Eggshell types and their evolutionary correlation with life-history strategies in squamates. PLoS One 10:e0138785
Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CED, Robinson BS, Hodgson DJ, Inger R (2018) A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6:e4794
Heulin B, Guillaume C-P, Bea A, Arrayago M (1993) Interprétation biogéographique de la bimodalité de reproduction du lézard Lacerta vivipara: un modèle pour l’étude de l’évolution de la viviparité. Biogeographica 69:1–11
Holtzman DA, Harris TW, Aranguren G, Bostock E (1999) Spatial learning of an escape task by young corn snakes, Elaphe guttata guttata. Anim Behav 57:51–60
Houston AI, McNamara JM (2014) Foraging currencies, metabolism and behavioural routines. J Anim Ecol 83:30–40
Huey RB (1982) Temperature, physiology, and the ecology of reptiles. In: Gans C, Pough FH (eds) Biology of the Reptilia. Academic Press, London, pp 25–91
Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Alvarez-Pérez HJ, Garland T (2009) Why tropical forest lizards are vulnerable to climate warming. Proc R Soc Lond B 276:1939–1948
Huey RB, Losos JB, Moritz C (2010) Are lizards toast? Science 328:832–833
Jolliffe IT (2002) Principal Component Analysis, 2nd edn. Springer-Verlag, New York
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest Package: tests in linear mixed effects models. J Stat Softw 82:1–26
LaDage LD, Roth TC, Cerjanic AM, Sinervo B, Pravosudov VV (2012) Spatial memory: are lizards really deficient? Biol Lett 8:939–941
Laird MK, Thompson MB, Whittington CM (2019) Facultative oviparity in a viviparous skink (Saiphos equalis). Biol Lett 15:20180827
Leach EC, Burwell CJ, Jones DN, Kitching RL (2018) Modelling the responses of Australian subtropical rainforest birds to changes in environmental conditions along elevational gradients. Austral Ecol 43:490–501
Li H, Elphick M, Shine R (2017) Potential targets for selection during the evolution of viviparity in cold-climate reptiles. Oecologia 183:21–30
Love OP, Williams TD (2008) The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am Nat 172:E135–E149
MacLean SA, Beissinger SR (2017) Species’ traits as predictors of range shifts under contemporary climate change: a review and meta-analysis. Glob Chang Biol 23:4094–4105
Marshall DJ, Uller T (2007) When is a maternal effect adaptive? Oikos 116:1957–1963
Meiri S, Bauer AM, Chirio L et al (2013) Are lizards feeling the heat? A tale of ecology and evolution under two temperatures. Glob Ecol Biogeogr 22:834–845
Mitchell TS, Janzen FJ, Warner DA (2018) Quantifying the effects of embryonic phenotypic plasticity on adult phenotypes in reptiles: A review of current knowledge and major gaps. J Exp Zool A 329:203–214
Mittelstaedt ML, Mittelstaedt H (1980) Homing by path integration in a mammal. Naturwissenschaften 67:566–567
Noble DWA, Carazo P, Whiting MJ (2012) Learning outdoors: male lizards show flexible spatial learning under semi-natural conditions. Biol Lett 8:946–948
Noble DWA, Stenhouse V, Schwanz LE (2018) Developmental temperatures and phenotypic plasticity in reptiles: a systematic review and meta-analysis. Biol Rev 93:72–97
Packard GC, Tracy CR, Roth JJ (1977) The physiological ecology of reptilian eggs and embryos, and the evolution of viviparity within the class Reptilia. Biol Rev 52:71–105
Painter D, Jennings DH, Moore MC (2002) Placental buffering of maternal steroid hormone effects on fetal and yolk hormone levels: a comparative study of a viviparous lizard, Sceloporus jarrovi, and an oviparous lizard, Sceloporus graciosus. Gen Comp Endocrinol 127:105–116
Pasquier G, Grüter C (2016) Individual learning performance and exploratory activity are linked to colony foraging success in a mass-recruiting ant. Behav Ecol 27:1702–1709
Pincheira-Donoso D, Tregenza T, Witt MJ, Hodgson DJ (2013) The evolution of viviparity opens opportunities for lizard radiation but drives it into a climatic cul-de-sac. Glob Ecol Biogeogr 22:857–867
Qualls CP, Andrews RM (1999) Cold climates and the evolution of viviparity in reptiles: cold incubation temperatures produce poor-quality offspring in the lizard, Sceloporus virgatus. Biol J Linn Soc 67:353–376
Qualls CP, Shine R (1998) Lerista bougainvillii, a case study for the evolution of viviparity in reptiles. J Evol Biol 11:63–78
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna Available from http://www.R-project.org
Radder RS, Elphick MJ, Warner DA, Pike DA, Shine R (2008) Reproductive modes in lizards: measuring fitness consequences of the duration of uterine retention of eggs. Funct Ecol 22:332–339
Revelle W (2017) psych: Procedures for psychological, psychometric, and personality research. Available from https://CRAN.R-project.org/package = psych
Schwenk K (1995) Of tongues and noses: chemoreception in lizards and snakes. Trends Ecol Evol 10:7–12
Sergeev M (1940) Researches in the viviparity of reptiles. Moscow Soc Nat (Jubilee Issue):1–34
Sheriff MJ, Love OP (2013) Determining the adaptive potential of maternal stress. Ecol Lett 16:271–280
Shine R (1985) The evolution of viviparity in reptiles: an ecological analysis. In: Gans C, Billett F (eds) Biology of the Reptilia. Wiley, New York, pp 605–694
Shine R (1995) A new hypothesis for the evolution of viviparity in reptiles. Am Nat 145:809–823
Shine R (2014) Evolution of an evolutionary hypothesis: a history of changing ideas about the adaptive significance of viviparity in reptiles. J Herpetol 48:147–161
Shine R, Harlow P (1993) Maternal thermoregulation influences offspring viability in a viviparous lizard. Oecologia 96:122–127
Shine R, Olsson M (2003) When to be born? Prolonged pregnancy or incubation enhances locomotor performance in neonatal lizards (Scincidae). J Evol Biol 16:823–832
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Sinervo B, Méndez-de-la-Cruz F, Miles DB et al (2010) Erosion of lizard diversity by climate change and altered thermal niches. Science 328:894–899
Siviter H, Deeming DC, Rosenberger J, Burman OHP, Moszuti SA, Wilkinson A (2017b) The impact of egg incubation temperature on the personality of oviparous reptiles. Anim Cogn 20:109–116
Siviter H, Deeming DC, van Giezen MFT, Wilkinson A (2017a) Incubation environment impacts the social cognition of adult lizards. R Soc Open Sci 4:170742
Siviter H, Deeming DC, Wilkinson A (2019) Egg incubation temperature influences the growth and foraging behaviour of juvenile lizards. Behav Process 165:9–13
Smith SA, Austin CC, Shine R (2001) A phylogenetic analysis of variation in reproductive mode within an Australian lizard Saiphos equalis, Scincidae. Biol J Linn Soc 74:131–139
Smith SA, Shine R (1997) Intraspecific variation in reproductive mode within the scincid lizard Saiphos equalis. Aust J Zool 45:435
Stewart JR, Mathieson AN, Ecay TW, Herbert JF, Parker SL, Thompson MB (2010) Uterine and eggshell structure and histochemistry in a lizard with prolonged uterine egg retention (Lacertilia, Scincidae, Saiphos). J Morphol 271:1342–1351
Sugiura N (1978) Further analysts of the data by Akaike’ s information criterion and the finite corrections. Commun Stat - Theor Method 7:13–26
Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB (2014) Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. P Natl Acad Sci USA 111:5610–5615
Telemeco RS, Elphick MJ, Shine R (2009) Nesting lizards (Bassiana duperreyi) compensate partly, but not completely, for climate change. Ecology 90:17–22
Tinkle D, Gibbons J (1977) The distribution and evolution of viviparity in reptiles. Misc Publ Mus Zool 154:1–55
Trnik M, Albrechtová J, Kratochvíl L (2011) Persistent effect of incubation temperature on stress-induced behavior in the Yucatan banded gecko (Coleonyx elegans). J Comp Psychol 125:22–30
Van Dyke JU, Brandley MC, Thompson MB (2014) The evolution of viviparity: molecular and genomic data from squamate reptiles advance understanding of live birth in amniotes. Reproduction 147:15–26
Verbeek MEM, Drent PJ, Wiepkema PR (1994) Consistent individual differences in early exploratory behaviour of male great tits. Anim Behav 48:1113–1121
Vince MA, Chinn S (1971) Effect of accelerated hatching on the initiation of standing and walking in the Japanese quail. Anim Behav 19:62–66
Visser ME (2008) Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc R Soc Lond B 275:649–659
Wang Z, Ma L, Shao M, Ji X (2017) Are viviparous lizards more vulnerable to climate warming because they have evolved reduced body temperature and heat tolerance? Oecologia 185:573–582
Webb GJW, Cooper-Preston H (1989) Effects of incubation temperature on crocodiles and the evolution of reptilian oviparity. Am Zool 29:953–971
Welberg LA, Seckl JR (2001) Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol 13:113–128
While GM, Uller T, Wapstra E (2009) Offspring performance and the adaptive benefits of prolonged pregnancy: experimental tests in a viviparous lizard. Funct Ecol 23:818–825
Wilkinson A, Chan H-M, Hall G (2007) Spatial learning and memory in the tortoise (Geochelone carbonaria). J Comp Psychol 121:412–418
Wu Q, Parker SL, Thompson MB (2009) Selected body temperature, metabolic rate and activity pattern of the Australian fossorial skink, Saiphos equalis. Herpetol J 19:127–133
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Acknowledgments
We thank Sergio Naretto and Cooper Van Der Waal for their assistance in the field and the laboratory, as well as Catarina Vila Pouca for her suggestions during the writing of the manuscript. We thank Camilla Whittington and Scott Parker for their advice regarding animal capture and identification. Finally, we are grateful to two anonymous reviewers for the comments which improved the manuscript.
Data availability statement
The datasets generated during the current study (raw data and R script code) are available through the Open Science Framework (OSF; https://osf.io/9zhmq/).
Funding
This work was supported by the Australasian Society for the Study of Animal Behaviour (2018 Student Grants) and the Australian Museum (2018/2019 Peter Rankin Trust Fund for Herpetology). This work was performed in the Plant Growth Facility (PGF) at Macquarie University. IB was supported by an international Research Training Program (iRTP) scholarship from the Australian Government and Macquarie University. VD and RL were supported by Le CROUS (Centre Régional des OEuvres Universitaires et Scolaires) de Strasbourg and University of Strasburg (France).
Author information
Authors and Affiliations
Contributions
Conceptualization: MJW and IB. Methodology: IB, VD, and RL. Formal analysis: IB. Resources: IB and MJW. Data curation: IB. Writing—original draft: IB, VD, and RL. Writing—review and editing: IB and MJW. Visualization: IB. Supervision: IB and MJW. Funding acquisition: IB and MJW.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Collection of animals was approved by the New South Wales National Parks and Wildlife Service, Office of Environment and Heritage (OEH; License No. SL101962). The experiments were approved by the Macquarie University Animal Ethics Committee (ARA 2017-029).
Additional information
Communicated by S. Joy Downes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beltrán, I., Loiseleur, R., Durand, V. et al. Effects of early thermal environment on the behavior and learning of a lizard with bimodal reproduction. Behav Ecol Sociobiol 74, 73 (2020). https://doi.org/10.1007/s00265-020-02849-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02849-6