Abstract
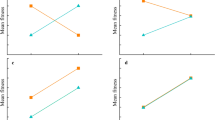
Previous studies have suggested that body size and locomotor performance are targets of Darwinian selection in reptiles. However, much of the variation in these traits may derive from phenotypically plastic responses to incubation temperature, rather than from underlying genetic variation. Intriguingly, incubation temperature may also influence cognitive traits such as learning ability. Therefore, we might expect correlations between a reptile’s size, locomotor speed and learning ability either due to selection on all of these traits or due to environmental effects during egg incubation. In the present study, we incubated lizard eggs (Scincidae: Bassiana duperreyi) under ‘hot’ and ‘cold’ thermal regimes and then assessed differences in hatchling body size, running speed and learning ability. We measured learning ability using a Y-maze and a food reward. We found high correlations between size, speed and learning ability, using two different metrics to quantify learning (time to solution, and directness of route), and showed that environmental effects (incubation temperature) cause these correlations. If widespread, such correlations challenge any simple interpretation of fitness advantages due to body size or speed within a population; for example, survivors may be larger and faster than nonsurvivors because of differences in learning ability, not because of their size or speed.





Similar content being viewed by others
References
Amiel JJ, Shine R (2012) Hotter nests produce smarter young lizards. Biol Lett 8(3):372–374. doi:10.1098/rsbl.2011.1161
Angilletta MJ Jr (2001) Variation in metabolic rate between populations of a geographically widespread lizard. Physiol Biochem Zool 74(1):11–21
Balasko M, Cabanac M (1998) Behavior of juvenile lizards (Iguana iguana) in a conflict between temperature regulation and palatable food. Brain Behav Evol 52(6):257–262
Burger J (1998) Antipredator behaviour of hatchling snakes: effects of incubation temperature and simulated predators. Anim Behav 56(3):547–553. doi:10.1006/anbe.1998.0809
Burghardt GM (1977) Learning processes in reptiles. In: Gans C, Tinkle DW (eds) Biology of the reptilia. Ecology and behaviour A, vol 7. Academic Press, London, pp 555–681
Cooper W (1994) Chemical discrimination by tongue-flicking in lizards: a review with hypotheses on its origin and its ecological and phylogenetic relationships. J Chem Ecol 20(2):439–487. doi:10.1007/bf02064449
Day LB, Crews D, Wilczynski W (1999) Spatial and reversal learning in congeneric lizards with different foraging strategies. Anim Behav 57(2):393–407
Deeming DC (2004) Reptilian incubation: environment, evolution and behaviour. Nottingham University Press, Sheffield
Dubey S, Shine R (2010) Evolutionary diversification of the lizard genus Bassiana (Scincidae) across Southern Australia. PLoS ONE 5(9):e12982. doi:10.1371/journal.pone.0012982
Dukas R (2004) Evolutionary biology of animal cognition. Annual review of ecology, evolution, and systematics 35 (ArticleType: research-article/Full publication date: 2004/Copyright © 2004 Annual Reviews):347–374
Elphick MJ, Shine R (1998) Longterm effects of incubation temperatures on the morphology and locomotor performance of hatchling lizards (Bassiana duperreyi, Scincidae). Biol J Linn Soc 63(3):429–447. doi:10.1111/j.1095-8312.1998.tb01527.x
Gamerman D, Lopes HF (2006) Markov chain Monte Carlo: stochastic simulation for Bayesian inference, vol 68. Chapman & Hall/CRC
Gelman A, Carlin JB, Stern HS, Rubin DB (2004) Bayesian data analysis. Chapman & Hall/CRC, Florida, USA
Grieco F, van Noordwijk AJ, Visser ME (2002) Evidence for the effect of learning on timing of reproduction in blue tits. Science 296(5565):136–138
Harlow PS (1996) A harmless technique for sexing hatchling lizards. Herpetol Rev 27:71–72
Irschick DJ, Herrel A, Vanhooydonck B, Huyghe K, Damme Rv (2005) Locomotor compensation creates a mismatch between laboratory and field estimates of escape speed in lizards: a cautionary tale for performance-to-fitness studies. Evolution 59(7):1579–1587. doi:10.1111/j.0014-3820.2005.tb01807.x
Janzen FJ (1993) An experimental analysis of natural selection on body size of hatchling turtles. Ecology 74(2):332–341
Lande R (1984) The genetic correlation between characters maintained by selection, linkage and inbreeding. Genet Res 44(03):309–320. doi:10.1017/S0016672300026549
Le Galliard J-F, Clobert J, Ferrière R (2004) Physical performance and Darwinian fitness in lizards. Nature 432(7016):502–505
Leal M, Powell BJ (2012) Behavioural flexibility and problem-solving in a tropical lizard. Biol Lett 8(1):28–30
Macphail EM (1982) Brain and intelligence in vertebrates. Oxford University Press, New York
Noble DW, Carazo P, Whiting MJ (2012) Learning outdoors: male lizards show flexible spatial learning under semi-natural conditions. Biol Lett 8(6):946–948
Radder RS, Quinn AE, Georges A, Sarre SD, Shine R (2008) Genetic evidence for co-occurrence of chromosomal and thermal sex-determining systems in a lizard. Biol Lett 4(2):176–178
Samaras TT (2007) Birthweight, height, brain size and intellectual ability. In: Samaras TT (ed) Human body size and the laws of scaling. Nova Science Publishers, New York, pp 301–318
Shettleworth SJ (2001) Animal cognition and animal behaviour. Anim Behav 61(2):277–286
Shettleworth SJ (2009) Cognition, evolution, and behaviour. Oxford University Press, New York
Shine R, Elphick MJ, Harlow PS (1997) The influence of natural incubation environments on the phenotypic traits of hatchling lizards. Ecology 78(8):2559–2568
Thomas DW, Blondel J, Perret P, Lambrechts MM, Speakman JR (2001) Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science 291(5513):2598–2600. doi:10.1126/science.1057487
Tomporowski P, Davis C, Miller P, Naglieri J (2008) Exercise and children’s intelligence, cognition, and academic achievement. Educ Psychol Rev 20(2):111–131. doi:10.1007/s10648-007-9057-0
Via S, Lande R (1985) Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39(3):505–522
Warner DA, Shine R (2007) Fitness of juvenile lizards depends on seasonal timing of hatching, not offspring body size. Oecologia 154(1):65–73
Wilkinson A, Huber L (2012) Cold-blooded cognition: reptilian cognitive abilities. In: Vonk J, Shackelford TK (eds) Oxford handbook of comparative evolutionary psychology. Oxford University Press, New York, pp 129–143
Wongwitdecha N, Alexander Marsden C (1996) Effects of social isolation rearing on learning in the morris water maze. Brain Res 715(1–2):119–124. doi:10.1016/0006-8993(95)01578-7
Acknowledgments
For funding, we thank the Australian Research Council, the Natural Sciences and Engineering Research Council of Canada and the Swedish Research Council (VR) and the University of Sydney. We thank Scott A. Sisson at the School of Mathematics and Statistics at the University of New South Wales for his comments on our statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amiel, J.J., Lindström, T. & Shine, R. Egg incubation effects generate positive correlations between size, speed and learning ability in young lizards. Anim Cogn 17, 337–347 (2014). https://doi.org/10.1007/s10071-013-0665-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-013-0665-4