Abstract
Purpose
Periacetabular bone loss poses a considerable challenge in the longevity and stability of acetabular implants used in total hip arthroplasty (THA). Innovations in implant design, specifically the introduction of three-dimensional (3D) porous titanium constructs, might reduce bone resorption. The purpose of this study was to build upon our previous randomized controlled trial, which found no change in periacetabular bone loss between a 3D porous none-hydroxyapatite coated titanium cup and a standard porous hydroxyapatite coated cup over a two year follow-up period by extending the follow-up duration to ten years post-surgery.
Methods
This was a single-centre, long-term follow-up study conducted over a ten year period in patients who had previously participated in a randomized controlled trial comparing a 3D porous titanium construct shell (PTC group) with a standard porous hydroxyapatite coated titanium shell (PC-group). The primary outcome measured was the change in bone mineral density (BMD) within four specific periacetabular zones, alongside overall bone loss, which was assessed through BMD in the lumbar spine at two, six and ten years postoperatively. Secondary outcomes included clinical outcome measures.
Results
In total, 18 in the PTC and 20 in the PC group were analysed for the primary endpoint up to ten years. The mean bone mineral density in zones 1–4 was 3.7% higher in the PTC group than in the PC group at six years postoperatively and 12.0% higher at ten years. Clinical outcomes, and the frequency of adverse events did not differ between the groups.
Conclusions
The PTC group displayed superior long-term bone preservation compared to the PC group while maintaining similar clinical outcomes up to ten years postoperatively. Although with a small sample size, our findings suggest that porous titanium cups have the potential to minimize BMD loss around the cup which could contribute to improving THA outcomes and implant durability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periacetabular bone loss represents a significant concern in the context of long-term stability and survivorship of acetabular implants utilized in total hip arthroplasty (THA) [1,2,3]. Factors such as periacetabular adaptive bone remodeling, commonly known as stress shielding, along with osteolysis induced by wear debris, exert substantial influence on periacetabular bone mass. Advancements in implant design have introduced three-dimensional (3D) porous titanium constructs integrated into acetabular shells, have been suggested to mitigate adaptive bone resorption and facilitate osseointegration in THA [4]. However, empirical evidence of the in-vivo effects of these innovations on human subjects remains limited [5]. The aim of this study was to serve as a long-term follow-up study building upon a randomized controlled trial comparing the performance of a 3D porous titanium construct shell (PTC) against a standard porous hydroxyapatite coated titanium shell (PC) at preventing bone mineral loss [6]. Our specific research questions were: how does PTC change periacetabular bone mineral density (BMD) during the ten years follow up? How does PTC and PC differ in terms of clinical outcome and complications?
Methods
Trial design
This study is a follow-up study of a single-centre randomized clinical trial [6]. The original trial aimed to investigate and compare the long-term effects of distinct acetabular implants used in THA on periacetabular bone density changes and clinical outcomes. Participants meeting inclusion criteria were recruited within a designated time frame (October 2009 to August 2013) at the Orthopaedic Department of Danderyd Hospital, in collaboration with the Department of Clinical Sciences at Karolinska Institute in Stockholm. The CONSORT statements were followed [7].
Participant selection and surgical procedure
We included individuals diagnosed with primary osteoarthritis, aged between 40 and 70 years, who were eligible for uncemented cups without significant structural acetabular defects and a femur type A or B according to Dorr [8]. Exclusions included prior hip surgeries on the affected side and regular use of corticosteroids, bisphosphonates or cytostatic drugs. A BMI of 35 or more was also set as an exclusion criterion. Patients provided informed consent before participation.
Patients allocated to the PTC group received an acetabular shell characterized by a 3D-printed porous titanium backside surface (Regenerex™-shell, E1™-liner, Biomet, USA) [9]. This implant featured a 1.5-mm thick trabecular-like porous titanium structure on its backside, with a porosity of 67% and an average pore size of 300 μm. The PC group underwent surgery with a titanium shell with hydroxyapatite (Pinnacle Duofix™-shell, Marathon™-liner, Depuy Johnson & Johnson, USA) [10], which comprised a porous coating composed of sintered titanium beads with an average pore size of 250 μm. Both liners utilized highly cross-linked polyethylene (HXLPE). On the femoral side, a 32-mm cobalt-chrome head was utilized alongside uncemented implants from either of the manufacturers (Bimetric™, Biomet, USA and Proxima™, Depuy Johnson & Johnson, USA). A posterolateral approach were used and all surgeries were performed by one of five experienced senior hip surgeons [11]. Postoperatively, patients were encouraged to use crutches according to individual preference during the initial weeks. Full weight-bearing was permitted as tolerated. Rehabilitation was supervised by a physiotherapist both during the hospital stay and subsequently in a day-clinic setting for the initial few weeks.
Endpoints and evaluation over extended intervals
The primary endpoint was change in BMD at the 6- and 10-year mark, with secondary endpoints focusing on functional clinical results. Follow-ups were scheduled at two, six and ten years postoperatively. All data was collected by research nurses who also acted as study coordinators. All data was collected and kept in Research Electronic data capture (REDCap), a digital case report form [12].
Bone densitometry
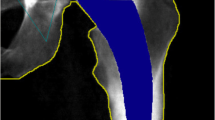
BMD within four specific zones, as outlined by Wilkinson et al. [13] and subsequently refined by Laursen et al. [14] (Fig. 1), was assessed in this study. The change in BMD within each zone was calculated by comparing the measured BMD values during follow-up examinations with the baseline value obtained two days after the surgery. This change was expressed as a percentage relative to the baseline measurement. To ensure consistency and accuracy, individual regions of interest (ROI) for each patient were saved and utilized for subsequent examinations, minimizing measurement errors.
BMD measurements from the lumbar spine at the level of L1-4 were also collected at the same time points as described above in order to assess overall bone loss and compare overall bone loss to BMD changes in the cup area. Only patients with complete BMD datasets for all follow ups were analyzed.
DXA scanning was conducted using a General Electric Healthcare machine, specifically the Lunar Prodigy Advance (GE Healthcare, Pittsburgh, US), operated with version 13.31.016 software. Rigorous quality controls of the DXA equipment were performed in adherence to the manufacturer’s guidelines throughout the study period, and no deviations from optimal functionality were identified.
Clinical outcome assessment
Clinical evaluations consisted of two self-administered scoring protocols, Harris hip score [15] and EQ-5D [16]. These were administered at each follow-up visits. Reoperation rate was followed by a review of the medical records and by asking patients at follow-up meetings whether they had gone through any revision surgery.
Statistical analysis
Emphasizing the significance of ROI 1 and 2, situated proximal to the cup, we considered compromised bone stock in these zones critical in the context of potential future cup revisions. We hypothesized that a mean difference of 10% with a standard deviation of 10% in bone mineral density within ROI 1 and 2 represented the smallest clinically relevant difference [13, 17]. Subjects with missing BMD data during follow-up visits were managed by employing the last observation carried forward method. This approach was used for individual follow-ups in both the PTC and PC groups. The study was analyzed according to the intention-to-treat principle. Given the normal distribution of both datasets, between-group comparisons were conducted using the unpaired Student t-test. All statistical analyses were performed using SPSS 22.0.
Ethics and registration
Regional ethics committee approval (number 2008/4:3) and ClinicalTrials.gov registration (number NCT01319227) was obtained to ensure adherence to ethical standards and transparent trial documentation.
Participant flow and baseline data
A total of 51 patients were enrolled (22 males and 29 females), mean age was 62 years. The baseline characteristics of both groups were similar. There was one death as a participant passed away from unrelated reasons five months after inclusion. 43 patients remained at the six years follow up and 38 at the ten year follow up (Table 1) (Fig. 2).
Results
Primary endpoint
At the 6-year follow-up the PTC group had a substantial increase (1.3%±1.4) in BMD. At the ten year follow-up the PTC group experienced a smaller reduction in BMD (-5.7% ±3.3) compared to the PC group (-17.7% ±2.9), (Table 2) (Fig. 3).
The overall bone loss in the cohort was − 0.009 g/cm2 as measured by BMD in the lumbar spine, L1-4. The PTC group did not lose significantly more BMD around the cup when compared to overall bone loss. The PC group lost significantly more BMD around the cup compared to overall bone loss (p = 0.002) (Table 3).
Secondary endpoint
The preoperative assessments for Harris Hip Score and EQ-5D score showed no significant differences between PTC group and the PC group. At the six year follow-up, both groups had improvements in Harris Hip Score and EQ-5D score compared to baseline. At the ten year follow-up, both groups exhibited improved functional outcomes compared to baseline. The Harris Hip Score and EQ-5D score showed no significant variations between the PTC group and the PC group (Table 4).
Adverse events
The PTC group had one case of dislocation, two cases of periprosthetic joint infection and one case of trochanteritis. Reoperations in this group included stem revision, and debridement, antibiotics and implant retention (DAIR) procedures, each performed once. The total number of reoperations and complications was also two and four, respectively. In the PC group, adverse events included one case each of dislocation, excessive subsidence/loosening of the stem, new fracture distal to stem (Vancouver C) and back pain. Additionally, this group underwent reoperations comprising of a single ORIF and a single stem revision. The total count of reoperations was two, with a total of four reported complications (Supp. Table 1).
Discussion
In this long-term follow-up of a randomized clinical trial, we found a lower decrease in periacetabular bone density in a none-hydroxyapatite coated PTC compared to hydroxyapatite coated PC. Our findings align with previous studies utilizing porous titanium acetabular cups which show favourable outcomes [18,19,20,21]. Our study is, to our knowledge, the only study that has examined long-term BMD changes and clinical outcomes of the use of PTC in THA.
Primary endpoint
We found significant differences in periacetabular BMD alterations between the PTC group and the PC group. The PC group exhibited considerable declines in BMD over the ten year follow-up period compared to the PTC group. The PTC group also showed less BMD loss when comparing overall bone loss using lumbar spine measurements. Our findings can be compared to the results of Massari et al. who found that porous titanium cups displayed stable BMD up to two years post operatively [22]. In similarity, Sodhi et al. found no radiographic failures of highly porous acetabular cups in primary THA with a minimum follow up of two years and seven months [23]. Although small, our study suggest that the PTC shells has the potential to mitigate long-term acetabular bone loss more effectively compared to PC shells. There are patient groups that could potentially benefit from the use of porous titanium cups, as uncemented hydroxyapatite coated porous cups in female patients with low systemic bone mineral density have been shown to be prone to cup migration [24]. Bone ingrowth and implant fixation might be further improved by variations in technical specifications such as pore size [25].
Secondary endpoint
Harris Hip Score and EQ-5D, revealed no significant differences between the PTC and PC groups at the six and ten year follow-ups. Both groups exhibited considerable improvements in functional outcomes compared to baseline.
Bone remodeling
Periacetabular bone remodeling is a well-documented phenomenon in THA, often characterized by a reduction in BMD in proximal regions and occasional minor reductions or even increases in distal areas, as reported in previous studies [17, 22, 26,27,28]. We found that the porous titanium cup exhibited a noticeable preservation of bone in zones 3 and 4. This observation might be explained by the increased porosity and trabecular-like geometry of the coating in this specific cup, potentially facilitating a deeper and more uniformly distributed bone ingrowth compared to the surface of the PC group. These features could explain the observed trend of higher BMD values measured in the distal zones.
The potential for a more robust shell fixation in zones 3 and 4 of the PTC might have resulted in reduced compression forces transmitted to the proximal periacetabular bone, contributing to the comparatively larger reduction in BMD observed in zone 1 of the PTC group [29]. Moreover, the phenomenon of more pronounced bone preservation in the distal two zones than in the proximal two could be attributed to a well-fixed shell inducing traction forces acting on the periacetabular bone distally which could stimulate an increase in bone mineral density, possibly explaining the BMD retention observed in zones 3 and 4. The proximity of the implanted metal shell to the native bone underlying the acetabular fossa could also have altered the load pattern in this zone, influencing BMD. Overall, these findings align with our initial arguments, reinforcing the potential role of the porous titanium cup’s design features in encouraging enhanced osseointegration and bone-preserving, particularly in the distal periacetabular zones, through mechanisms such as improved bone ingrowth and altered load patterns.
Adverse events
Both the PTC and PC groups exhibited similar adverse event rates and subsequent reoperations. Each group reported four adverse events, including issues like dislocation, subsidence/loosening of the stem, prosthesis joint infection, back pain, and trochanteritis. The comparable incidence of adverse events suggests a similar safety profile for both implant types, although the number of adverse events were few.
Limitations
There are some limitations to our study. There was a relatively small sample size and there is a need for larger cohorts to validate our findings. Additionally, the evaluation focused on specific BMD changes and functional outcomes, necessitating a comprehensive assessment of implant longevity and survivorship. Furthermore, the presence of compromised bone stock can influence implant performance and outcomes in THA. While our cohort did not include individuals with deficient bone stock, it is important to note that implant selection in cases of poor bone quality may yield different results in terms of bone remodeling and clinical outcomes.
Conclusion
In conclusion, our study suggests a role for PTC in preserving periacetabular bone density following THA. We found that the porous titanium cup without hydroxyapatite has advantages in mitigating long-term acetabular bone loss compared to a standard hydroxyapatite coated porous cup while maintaining clinical outcomes. Further research in larger cohorts with long-term follow-up is warranted to corroborate these findings.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Thanner J, Kärrholm J, Malchau H, Herberts P (1999) Poor outcome of the PCA and Harris-Galante hip prostheses. Randomized study of 171 arthroplasties with 9-year follow-up. Acta Orthop Scand 70:155–162. https://doi.org/10.3109/17453679909011255
Hallan G, Lie SA, Havelin LI (2006) High wear rates and extensive osteolysis in 3 types of uncemented total hip arthroplasty: a review of the PCA, the Harris Galante and the Profile/Tri-Lock Plus arthroplasties with a minimum of 12 years median follow-up in 96 hips. Acta Orthop 77:575–584. https://doi.org/10.1080/17453670610012638
Mancino F, Cacciola G, Di Matteo V et al (2020) Reconstruction options and outcomes for acetabular bone loss in revision hip arthroplasty. Orthop Rev (Pavia) 12:8655. https://doi.org/10.4081/or.2020.8655
Lim L, Bobyn JD, Bobyn KM et al (2012) The Otto Aufranc Award: demineralized bone matrix around porous implants promotes rapid gap healing and bone ingrowth. Clin Orthop Relat Res 470:357–365. https://doi.org/10.1007/s11999-011-2011-y
Lindgren V, Galea VP, Nebergall A et al (2018) Radiographic and Clinical outcomes of Porous Titanium-Coated and plasma-sprayed Acetabular shells: a five-year prospective Multicenter Study. J Bone Joint Surg Am 100:1673–1681. https://doi.org/10.2106/JBJS.17.00729
Salemyr M, Muren O, Eisler T et al (2015) Porous titanium construct cup compared to porous coated titanium cup in total hip arthroplasty. A randomised controlled trial. Int Orthop (SICOT) 39:823–832. https://doi.org/10.1007/s00264-014-2571-z
Schulz KF, Altman DG, Moher D, the CONSORT Group (2010) CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med 8:18. https://doi.org/10.1186/1741-7015-8-18
Dorr LD, Faugere MC, Mackel AM et al (1993) Structural and cellular assessment of bone quality of proximal femur. Bone 14:231–242. https://doi.org/10.1016/8756-3282(93)90146-2
Zimmer BioMet Regenerex® Porous Titanium Construct
PINNACLE™ Acetabular Cup System | DePuy Synthes. In: J&J MedTech. https://www.jnjmedtech.com/en-EMEA/product/pinnacle-acetabular-cup-system. Accessed 12 Dec 2023
Ait Mokhtar M (2020) Postero-posterolateral approach in total hip arthroplasty. Int Orthop 44:2577–2585. https://doi.org/10.1007/s00264-020-04679-7
Harris PA, Taylor R, Thielke R et al (2009) Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf 42:377–381. https://doi.org/10.1016/j.jbi.2008.08.010
Wilkinson JM, Peel NF, Elson RA et al (2001) Measuring bone mineral density of the pelvis and proximal femur after total hip arthroplasty. J Bone Joint Surg Br 83:283–288. https://doi.org/10.1302/0301-620x.83b2.10562
Laursen MB, Nielsen PT, Søballe K (2005) DXA scanning of acetabulum in patients with cementless total hip arthroplasty. J Clin Densitom 8:476–483. https://doi.org/10.1385/jcd:8:4:476
Nilsdotter A, Bremander A (2011) Measures of hip function and symptoms: Harris Hip score (HHS), hip disability and osteoarthritis outcome score (HOOS), Oxford Hip score (OHS), Lequesne Index of Severity for Osteoarthritis of the hip (LISOH), and American Academy of Orthopedic Surgeons (AAOS) hip and knee questionnaire. Arthritis Care Res (Hoboken) 63(Suppl 11):S200–207. https://doi.org/10.1002/acr.20549
Rabin R, de Charro F (2001) EQ-5D: a measure of health status from the EuroQol Group. Ann Med 33:337–343. https://doi.org/10.3109/07853890109002087
Baad-Hansen T, Kold S, Nielsen PT et al (2011) Comparison of trabecular metal cups and titanium fiber-mesh cups in primary hip arthroplasty. Acta Orthop 82:155–160. https://doi.org/10.3109/17453674.2011.572251
Miyagawa T, Matsumoto K, Komura S, Akiyama H (2021) Total hip arthroplasty using a three-dimensional porous titanium acetabular cup: an examination of micromotion using subject-specific finite element analysis. BMC Musculoskelet Disord 22:308. https://doi.org/10.1186/s12891-021-04174-z
Shichman I, Somerville L, Lutes WB et al (2022) Outcomes of novel 3D-printed fully porous titanium cup and a cemented highly cross-linked polyethylene liner in complex and revision total hip arthroplasty. Arthroplasty 4:51. https://doi.org/10.1186/s42836-022-00152-5
Delanois RE, Gwam CU, Mohamed N et al (2017) Midterm outcomes of Revision Total Hip Arthroplasty with the Use of a Multihole highly-porous Titanium Shell. J Arthroplast 32:2806–2809. https://doi.org/10.1016/j.arth.2017.03.065
Berlinberg EJ, Kavian JA, Roof MA et al (2022) Minimum 2-Year outcomes of a novel 3D-printed fully porous Titanium Acetabular Shell in Revision Total Hip Arthroplasty. Arthroplasty Today 18:39–44. https://doi.org/10.1016/j.artd.2022.08.007
Massari L, Bistolfi A, Grillo PP et al (2017) Periacetabular bone densitometry after total hip arthroplasty with highly porous titanium cups: a 2-year follow-up prospective study. Hip Int 27:551–557. https://doi.org/10.5301/hipint.5000509
Sodhi N, Khlopas A, Berliner Z et al (2019) Survivorship and Radiographic Analysis of highly porous Acetabular cups designed for Improved Osseointegration potential. Surg Technol Int 34:425–429
Finnilä S, Moritz N, SvedströM E et al (2016) Increased migration of uncemented acetabular cups in female total hip arthroplasty patients with low systemic bone mineral density. Acta Orthop 87:48–54. https://doi.org/10.3109/17453674.2015.1115312
Taniguchi N, Fujibayashi S, Takemoto M et al (2016) Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: an in vivo experiment. Mater Sci Eng C Mater Biol Appl 59:690–701. https://doi.org/10.1016/j.msec.2015.10.069
Digas G, Kärrholm J, Thanner J (2006) Different loss of BMD using uncemented press-fit and whole polyethylene cups fixed with cement: repeated DXA studies in 96 hips randomized to 3 types of fixation. Acta Orthop 77:218–226. https://doi.org/10.1080/17453670610045948
Penny JO, Brixen K, Varmarken JE et al (2012) Changes in bone mineral density of the acetabulum, femoral neck and femoral shaft, after hip resurfacing and total hip replacement: two-year results from a randomised study. J Bone Joint Surg Br 94:1036–1044. https://doi.org/10.1302/0301-620X.94B8.28222
Anderl C, Steinmair M, Hochreiter J (2022) Bone preservation in total hip arthroplasty. J Arthroplast 37:1118–1123. https://doi.org/10.1016/j.arth.2022.01.077
Schmidt R, Kress AM, Nowak M et al (2012) Periacetabular cortical and cancellous bone mineral density loss after press-fit cup fixation: a prospective 7-year follow-up. J Arthroplasty 27:1358–1363e1. https://doi.org/10.1016/j.arth.2011.09.031
Funding
Open access funding provided by Karolinska Institute. We extend our gratitude for the funding support received from various foundations, including the Ulla and Gustaf Ugglas Stiftelse, Åke Wiberg Stiftelse, Loo and Hans Ostermans Stiftelse, Sven Norén Foundation, and the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet.
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
Conceptualization: MS, OS, Data curation: MA, MS, SM, MM, OS. Formal analysis: MA, SM, OS. Funding acquisition: OS. Investigation: MS, OS. Methodology: MA, OS. Project administration: MS, OS. Resources: OS. Software: MA. Supervision: OS. Validation: MS, OS. Visualization: MA. Writing – original draft: MA. Writing – review & editing: MS, SM, MM, OS.
Corresponding author
Ethics declarations
Ethics approval
The study adhered to the ethical guidelines outlined in the Helsinki Declaration and received approval from the local ethics committee (number 2008/4:3). The trial is registered at ClinicalTrials.gov (number NCT01319227).
Consent to participate
Not applicable.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Axenhus, M., Salemyr, M., Mukka, S. et al. Long-term follow-up of bone density changes in total hip arthroplasty: comparative analysis from a randomized controlled trial of a porous titanium construct shell vs. a porous coated shell. International Orthopaedics (SICOT) (2024). https://doi.org/10.1007/s00264-024-06289-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00264-024-06289-z