Abstract
Background
Hypercholesterolemia is one of the risk factors for colorectal cancer (CRC). Cholesterol can participate in the regulation of human T cell function and affect the occurrence and development of CRC.
Objective
To elucidate the pathogenesis of CRC immune escape mediated by CD8+ T cell exhaustion induced by cholesterol.
Methods
CRC samples (n = 217) and healthy individuals (n = 98) were recruited to analyze the relationship between peripheral blood cholesterol levels and the clinical features of CRC. An animal model of CRC with hypercholesterolemia was established. Intraperitoneal intervention with endoplasmic reticulum stress (ERS) inhibitors in hypercholesterolemic CRC mice was performed. CD69, PD1, TIM-3, and CTLA-4 on CD8+ T cells of spleens from C57BL/6 J mice were detected by flow cytometry. CD8+ T cells were cocultured with MC38 cells (mouse colon cancer cell line). The proliferation, apoptosis, migration and invasive ability of MC38 cells were detected by CCK-8 assay, Annexin-V APC/7-AAD double staining, scratch assay and transwell assay, respectively. Transmission electron microscopy was used to observe the ER structure of CD8+ T cells. Western blotting was used to detect the expression of ERS and mitophagy-related proteins. Mitochondrial function and energy metabolism were measured. Immunoprecipitation was used to detect the interaction of endoplasmic reticulum-mitochondria contact site (ERMC) proteins. Immunofluorescence colocalization was used to detect the expression and intracellular localization of ERMC-related molecules.
Results
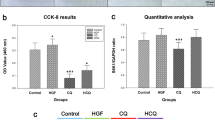
Peripheral blood cholesterol-related indices, including Tc, low density lipoproteins (LDL) and Apo(a), were all increased, and high density lipoprotein (HDL) was decreased in CRCs. The proliferation, migration and invasion abilities of MC38 cells were enhanced, and the proportion of tumor cell apoptosis was decreased in the high cholesterol group. The expression of IL-2 and TNF-α was decreased, while IFN-γ was increased in the high cholesterol group. It indicated high cholesterol could induce exhaustion of CD8+ T cells, leading to CRC immune escape. Hypercholesterolemia damaged the ER structure of CD8+ T cells and increased the expression of ER stress molecules (CHOP and GRP78), lead to CD8+ T cell exhaustion. The expression of mitophagy-related proteins (BNIP3, PINK and Parkin) in exhausted CD8+ T cells increased at high cholesterol levels, causing mitochondrial energy disturbance. High cholesterol enhanced the colocalization of Fis1/Bap31, MFN2/cox4/HSP90B1, VAPB/PTPIP51, VDAC1/IPR3/GRP75 in ERMCs, indicated that high cholesterol promoted the intermolecular interaction between ER and mitochondrial membranes in CD8+ T cells.
Conclusion
High cholesterol regulated the ERS-ERMC-mitophagy axis to induce the exhaustion of CD8+ T cells in CRC.




Similar content being viewed by others
Abbreviations
- CI:
-
Confidence interval
- CTLs:
-
Cytotoxic T lymphocytes
- DCA:
-
Deoxycholic acid
- Dvl:
-
Dishevelled
- ERMCs:
-
Endoplasmic reticulum-mitochondria contact sites
- ERS:
-
Endoplasmic reticulum stress
- HDL-C:
-
High‐density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- LDLR:
-
Low-density lipoprotein receptor
- LXRs:
-
Liver X receptors
- MACS:
-
Magnetic cell separation
- MMP:
-
Mitochondrial membrane potential
- NHL:
-
Non-Hodgkin lymphoma
- OCR:
-
Oxygen consumption rate
- SREBP2:
-
Sterol regulatory element-binding protein 2
- SQLE:
-
Squalene epoxidase
- TCR:
-
T-cell receptor
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71(1):7–33
Zhang L, Cao F, Zhang G, Shi L, Chen S, Zhang Z, Zhi W, Ma T (2019) Trends in and predictions of colorectal cancer incidence and mortality in China From 1990 to 2025. Front Oncol 9:98
Jun SY, Brown AJ, Chua NK, Yoon JY, Lee JJ, Yang JO, Jang I, Jeon SJ, Choi TI, Kim CH et al (2021) Reduction of squalene epoxidase by cholesterol accumulation accelerates colorectal cancer progression and metastasis. Gastroenterology 160(4):1194-1207.e1128
Cornish AJ, Law PJ, Timofeeva M, Palin K, Farrington SM, Palles C, Jenkins MA, Casey G, Brenner H, Chang-Claude J et al (2020) Modifiable pathways for colorectal cancer: a mendelian randomisation analysis. Lancet Gastroenterol Hepatol 5(1):55–62
Wang Y, Sun XQ, Lin HC, Wang DS, Wang ZQ, Shao Q, Wang FH, Yan SM, Liang JY, Zeng ZL et al (2019) Correlation between immune signature and high-density lipoprotein cholesterol level in stage II/III colorectal cancer. Cancer Med 8(3):1209–1217
Mayengbam SS, Singh A, Pillai AD, Bhat MK (2021) Influence of cholesterol on cancer progression and therapy. Transl Oncol 14(6):101043
Tanaka T, Oyama T, Sugie S, Shimizu M (2016) Different susceptibilities between Apoe- and Ldlr-deficient mice to inflammation-associated colorectal carcinogenesis. Int J Mol Sci 17(11):1806
Barbera NA, Minke B, Levitan I (2019) Comparative docking analysis of cholesterol analogs to ion channels to discriminate between stereospecific binding vs. stereospecific response. Channels (Austin) 13(1):136–146
Narwal V, Deswal R, Batra B, Kalra V, Hooda R, Sharma M, Rana JS (2019) Cholesterol biosensors: a review. Steroids 143:6–17
Luo J, Yang H, Song BL (2020) Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol 21(4):225–245
Hu J, La Vecchia C, de Groh M, Negri E, Morrison H, Mery L (2012) Dietary cholesterol intake and cancer. Ann Oncol 23(2):491–500
Zhang KL, Zhu WW, Wang SH, Gao C, Pan JJ, Du ZG, Lu L, Jia HL, Dong QZ, Chen JH et al (2021) Organ-specific cholesterol metabolic aberration fuels liver metastasis of colorectal cancer. Theranostics 11(13):6560–6572
Sheng R, Kim H, Lee H, Xin Y, Chen Y, Tian W, Cui Y, Choi JC, Doh J, Han JK et al (2014) Cholesterol selectively activates canonical Wnt signalling over non-canonical Wnt signalling. Nat Commun 5:4393
He L, Li H, Pan C, Hua Y, Peng J, Zhou Z, Zhao Y, Lin M (2021) Squalene epoxidase promotes colorectal cancer cell proliferation through accumulating calcitriol and activating CYP24A1-mediated MAPK signaling. Cancer Commun (Lond) 41(8):726–746
Louis P, Hold GL, Flint HJ (2014) The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 12(10):661–672
Li C, Wang Y, Liu D, Wong CC, Coker OO, Zhang X, Liu C, Zhou Y, Liu Y, Kang W et al (2022) Squalene epoxidase drives cancer cell proliferation and promotes gut dysbiosis to accelerate colorectal carcinogenesis. Gut 71:2253–2265
Cao H, Xu M, Dong W, Deng B, Wang S, Zhang Y, Wang S, Luo S, Wang W, Qi Y et al (2017) Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int J Cancer 140(11):2545–2556
Lucken-Ardjomande S, Montessuit S, Martinou JC (2008) Bax activation and stress-induced apoptosis delayed by the accumulation of cholesterol in mitochondrial membranes. Cell Death Differ 15(3):484–493
Spann NJ, Glass CK (2013) Sterols and oxysterols in immune cell function. Nat Immunol 14(9):893–900
Traversari C, Sozzani S, Steffensen KR, Russo V (2014) LXR-dependent and -independent effects of oxysterols on immunity and tumor growth. Eur J Immunol 44(7):1896–1903
Yuan J, Cai T, Zheng X, Ren Y, Qi J, Lu X, Chen H, Lin H, Chen Z, Liu M et al (2021) Potentiating CD8(+) T cell antitumor activity by inhibiting PCSK9 to promote LDLR-mediated TCR recycling and signaling. Protein Cell 12(4):240–260
Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H (2001) Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 61(13):5132–5136
Wu W, Shi X, Xu C (2016) Regulation of T cell signalling by membrane lipids. Nat Rev Immunol 16(11):690–701
Li M, Yang Y, Wei J, Cun X, Lu Z, Qiu Y, Zhang Z, He Q (2018) Enhanced chemo-immunotherapy against melanoma by inhibition of cholesterol esterification in CD8(+) T cells. Nanomedicine 14(8):2541–2550
Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, Meng X, Li L, Wang J, Xu C et al (2016) Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature 531(7596):651–655
Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, Wang Q, Yang M, Kalady MF, Qian J et al (2019) Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab 30(1):143-156.e145
Yang X, Qi Q, Pan Y, Zhou Q, Wu Y, Zhuang J, Xu J, Pan M, Han S (2020) Single-cell analysis reveals characterization of infiltrating t cells in moderately differentiated colorectal cancer. Front Immunol 11:620196
Dong L, Yang X, Wang Y, Jin Y, Zhou Q, Chen G, Han S (2021) Key markers involved in the anticolon cancer response of cd8+ t cells through the regulation of cholesterol metabolism. J Oncol 2021:9398661
Weiser MR (2018) AJCC 8th edition: colorectal cancer. Ann Surg Oncol 25(6):1454–1455. https://doi.org/10.1245/s10434-018-6462-1
Schlimmer N, Kratz M, Böhm M, Baumhäkel M (2011) Telmisartan, ramipril and their combination improve endothelial function in different tissues in a murine model of cholesterol-induced atherosclerosis. Br J Pharmacol 163(4):804–814
Ikeda SI, Kurihara T, Jiang X, Miwa Y, Lee D, Serizawa N, Jeong H, Mori K, Katada Y, Kunimi H et al (2022) Scleral PERK and ATF6 as targets of myopic axial elongation of mouse eyes. Nat Commun 13(1):5859
Kurien BT, Scofield RH (2006) Western blotting. Methods 38(4):283–293
Wu Y, Li Q, Chen XZ (2007) Detecting protein-protein interactions by Far western blotting. Nat Protoc 2(12):3278–3284
Xi Y, Yani Z, Jing M, Yinhang W, Xiaohui H, Jing Z, Quan Q, Shuwen H (2021) Mechanisms of induction of tumors by cholesterol and potential therapeutic prospects. Biomed Pharmacother 144:112277
Eftekhari A, Ahmadian E, Salatin S, Sharifi S, Dizaj SM, Khalilov R, Hasanzadeh M (2019) Current analytical approaches in diagnosis of melanoma. Trac Trends Anal Chem 116:122–135
Li D, Li Y, Hernandez JA, Patenia R, Kim TK, Khalili J, Dougherty MC, Hanley PJ, Bollard CM, Komanduri KV et al (2010) Lovastatin inhibits T-cell proliferation while preserving the cytolytic function of EBV, CMV, and MART-1-specific CTLs. J Immunother 33(9):975–982
Overton ET, Sterrett S, Westfall AO, Kahan SM, Burkholder G, Zajac AJ, Goepfert PA, Bansal A (2014) Effects of atorvastatin and pravastatin on immune activation and T-cell function in antiretroviral therapy-suppressed HIV-1-infected patients. AIDS 28(17):2627–2631
Chern YJ, Wong JCT, Cheng GSW, Yu A, Yin Y, Schaeffer DF, Kennecke HF, Morin G, Tai IT (2019) The interaction between SPARC and GRP78 interferes with ER stress signaling and potentiates apoptosis via PERK/eIF2α and IRE1α/XBP-1 in colorectal cancer. Cell Death Dis 10(7):504
Schröder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74:739–789
Sozen E, Ozer NK (2017) Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: an updated mini-review. Redox Biol 12:456–461
Zhou J, Werstuck GH, Lhoták S, de Koning AB, Sood SK, Hossain GS, Møller J, Ritskes-Hoitinga M, Falk E, Dayal S et al (2004) Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation 110(2):207–213
Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G et al (2007) Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation 116(11):1226–1233
Hurst KE, Lawrence KA, Essman MT, Walton ZJ, Leddy LR, Thaxton JE (2019) Endoplasmic reticulum stress contributes to mitochondrial exhaustion of CD8(+) T cells. Cancer Immunol Res 7(3):476–486
Cao Y, Trillo-Tinoco J, Sierra RA, Anadon C, Dai W, Mohamed E, Cen L, Costich TL, Magliocco A, Marchion D et al (2019) ER stress-induced mediator C/EBP homologous protein thwarts effector T cell activity in tumors through T-bet repression. Nat Commun 10(1):1280
Lee BR, Chang SY, Hong EH, Kwon BE, Kim HM, Kim YJ, Lee J, Cho HJ, Cheon JH, Ko HJ (2014) Elevated endoplasmic reticulum stress reinforced immunosuppression in the tumor microenvironment via myeloid-derived suppressor cells. Oncotarget 5(23):12331–12345
Chodari L, Dilsiz Aytemir M, Vahedi P, Alipour M, Vahed SZ, Khatibi SMH, Ahmadian E, Ardalan M, Eftekhari A (2021) Targeting mitochondrial biogenesis with polyphenol compounds. Oxid Med Cell Longev 2021:4946711
Lebeau J, Saunders JM, Moraes VWR, Madhavan A, Madrazo N, Anthony MC, Wiseman RL (2018) The PERK arm of the unfolded protein response regulates mitochondrial morphology during acute endoplasmic reticulum stress. Cell Rep 22(11):2827–2836
Bouman L, Schlierf A, Lutz AK, Shan J, Deinlein A, Kast J, Galehdar Z, Palmisano V, Patenge N, Berg D et al (2011) Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ 18(5):769–782
Killackey SA, Philpott DJ, Girardin SE (2020) Mitophagy pathways in health and disease. J Cell Biol 219(11):e202004029
Palikaras K, Lionaki E, Tavernarakis N (2018) Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol 20(9):1013–1022
Yoo SM, Jung YK (2018) A molecular approach to mitophagy and mitochondrial dynamics. Mol Cells 41(1):18–26
Fan Y, Simmen T (2019) Mechanistic connections between endoplasmic reticulum (ER) redox control and mitochondrial metabolism. Cells 8(9):1071
Namgaladze D, Khodzhaeva V, Brüne B (2019) ER-mitochondria communication in cells of the innate immune system. Cells 8(9):1088
Wang N, Wang C, Zhao H, He Y, Lan B, Sun L, Gao Y (2021) The MAMs structure and its role in cell death. Cells 10(3):657
Namba T (2019) BAP31 regulates mitochondrial function via interaction with Tom40 within ER-mitochondria contact sites. Sci Adv 5(6):eaaw1386
Giamogante F, Barazzuol L, Brini M, Calì T (2020) ER-mitochondria contact sites reporters: strengths and weaknesses of the available approaches. Int J Mol Sci 21(21):8157
Yu R, Jin SB, Lendahl U, Nistér M, Zhao J (2019) Human Fis1 regulates mitochondrial dynamics through inhibition of the fusion machinery. Embo j 38(8):e99748
Hu Y, Chen H, Zhang L, Lin X, Li X, Zhuang H, Fan H, Meng T, He Z, Huang H et al (2021) The AMPK-MFN2 axis regulates MAM dynamics and autophagy induced by energy stresses. Autophagy 17(5):1142–1156
Funding
This research was supported by Public Welfare Technology Application Research Program of Huzhou (No.2021GY05), Medical and Health Technology Project of Zhejiang Province (No.2022KY1220, No.2021KY343) and Zhejiang Provincial Natural Science Foundation (No. LQ23H160006).
Author information
Authors and Affiliations
Contributions
YX and HS conceived of the study. YX and WY wrote the manuscript. YQ and LJ performed the experiments. ZJ drew the figures. QQ, JY and JY analyzed the data. All authors read and approved the paper.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that no conflicts of interest exist.
Ethical approval and consent to participate
Healthy individuals and CRC samples at Huzhou Central Hospital from January 2019 to December 2020 were recruited for this study. This study was approved by the Ethics Committee of Huzhou Central Hospital (No. 20191101-02).
Consent for publication
All patients volunteered to participate in the study and signed a written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
262_2023_3555_MOESM1_ESM.tif
Mitochondrial dysfunction in high cholesterol-induced exhausted CD8+ T cells. A, workflow of this part. B, mitochondrial function indices of CD8+ T cells in the normal cholesterol group, high cholesterol group, normal cholesterol colorectal cancer group, and high cholesterol colorectal cancer group: Mitochondrial membrane potential, ATP content, OCR value (cell oxygen respiration O2 consumption rate), ROS relative content, acetyl coenzyme A, NADH/NAD + , and mitochondrial free calcium. C, CD8+ T cells in the high-cholesterol colorectal cancer group were saved by ADP. Comparison of changes in the following mitochondrial functional indicators: mitochondrial membrane potential, ATP content, OCR value (O2 consumption rate in cell aerobic respiration), ROS relative content, acetyl coenzyme A, and NADH/NAD + . D, CD8+ T cells in the high-cholesterol colorectal cancer group were treated with ADP for energy rescue. Flow cytometry was used to detect the expression of the irritant receptor CD69 and inhibitory receptors PD-1, CTLA-4 and TIM- in CD8+ T cells before and after energy rescue. E, the expression levels of the mitophagy-related proteins BINP3, PINK1 and Parkin in CD8+ T cells from mice in the normal cholesterol group, high cholesterol group, normal cholesterol colorectal cancer group and high cholesterol CRC group significantly differed. * indicates P < 0.05, ** indicates P < 0.01, *** indicates P < 0.001 (TIF 3171 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shuwen, H., Yinhang, W., Jing, Z. et al. Cholesterol induction in CD8+ T cell exhaustion in colorectal cancer via the regulation of endoplasmic reticulum-mitochondria contact sites. Cancer Immunol Immunother 72, 4441–4456 (2023). https://doi.org/10.1007/s00262-023-03555-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03555-8