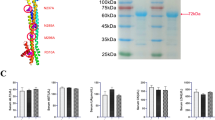
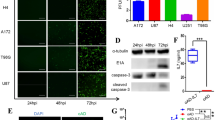
Abstract
Even with progressive combination treatments, the prognosis of patients with glioblastoma (GBM) remains extremely poor. OV is one of the new promising therapeutic strategies to treat human GBM. OVs stimulate immune cells to release cytokines such as IFN-γ during oncolysis, further improve tumor microenvironment (TME) and enhance therapeutic efficacy. IFN-γ plays vital role in the apoptosis of tumor cells and recruitment of tumor-infiltrating T cells. We hypothesized that oncolytic herpes simplex virus-1 (oHSV-1) enhanced the antitumor efficacy of novel CD70-specific chimeric antigen receptor (CAR) T cells by T cell infiltration and IFN-γ release. In this study, oHSV-1 has the potential to stimulate IFN-γ secretion of tumor cells rather than T cell secretion and lead to an increase of T cell activity, as well as CD70-specific CAR T cells can specifically recognize and kill tumor cells in vitro. Specifically, combinational therapy with CD70-specific CAR T and oHSV-1 promotes tumor degradation by enhancing pro-inflammatory circumstances and reducing anti-inflammatory factors in vitro. More importantly, combined therapy generated potent antitumor efficacy, increased the proportion of T cells and natural killer cells in TME, and reduced regulatory T cells and transformed growth factor-β1 expression in orthotopic xenotransplanted animal model of GBM. In summary, we reveal that oHSV-1 enhance the therapeutic efficacy of CD70-spefific CAR T cells by intratumoral T cell infiltration and IFN-γ release, supporting the use of CAR T therapy in GBM therapeutic strategies.







Similar content being viewed by others
Availability of data and materials
All data generated in this study are available from the corresponding author on reasonable request.
Abbreviations
- CAR:
-
Chimeric antigen receptor
- DAPI:
-
2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride.
- DMEM:
-
Dulbecco's modified eagle medium
- EGFP:
-
Enhanced green fluorescent protein
- GBM:
-
Glioblastoma
- IF:
-
Immunofluorescence
- IFN:
-
Interferon
- IL:
-
Interleukin
- IVIS:
-
In vivo imaging system
- NK:
-
Natural killer
- PBMCs:
-
Peripheral blood mononuclear cells
- PBS:
-
Phosphate buffer saline
- oHSV-1:
-
Oncolytic herpes simplex virus-1
- OVs:
-
Oncolytic virus
- TGF:
-
Transforming growth factor
- TME:
-
Tumor microenvironment
- TNF:
-
Tumor necrosis factor
- trCD27:
-
Truncated CD27
- Treg:
-
Regulatory T
- SME:
-
Scanning electron microscopy
References
Migliorini D, Dietrich P Y, Stupp R, Linette G P, Posey A D, Jr., June C H (2018) CAR T-Cell therapies in glioblastoma: a first look. Clin Cancer Res. 24: 535-540.
Seystahl K, Wick W, Weller M (2016) Therapeutic options in recurrent glioblastoma–an update. Crit Rev Oncol Hematol 99:389–408
Zhang Q, Liu F (2020) Advances and potential pitfalls of oncolytic viruses expressing immunomodulatory transgene therapy for malignant gliomas. Cell Death Dis 11:485
Lichty BD, Breitbach CJ, Stojdl DF, Bell JC (2014) Going viral with cancer immunotherapy. Nat Rev Cancer 14:559–567
Kaufman HL, Kohlhapp FJ, Zloza A (2016) Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 15:660
Twumasiboateng K, Pettigrew JL, Kwok YYE, Bell JC, Nelson BH (2018) Oncolytic viruses as engineering platforms for combination immunotherapy. Nat Rev Cancer 18:419–432
Marelli G, Howells A, Lemoine NR, Wang Y (2018) Oncolytic viral therapy and the immune system: a double-edged sword against cancer. Front Immunol 9:866
Bommareddy PK, Shettigar M, Kaufman HL (2018) Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol 18:498–513
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR et al (2013) Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368:1509–1518
O'Rourke D M, Nasrallah M P, Desai A, Melenhorst J J, Mansfield K, Morrissette J J D, et al. (2017) A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 9
Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G et al (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169:2756–2761
Mukherjee P, Ginardi AR, Madsen CS, Tinder TL, Jacobs F, Parker J et al (2001) MUC1-specific CTLs are non-functional within a pancreatic tumor microenvironment. Glycoconj J 18:931–942
Moon EK, Wang L, Dolfi DV, Wilson CB, Ranganathan R, Sun J et al (2014) Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res 20:4262–4273
Beatty GL, Ohara MH (2016) Chimeric antigen receptor-modified T cells for the treatment of solid tumors: defining the challenges and next steps. Pharmacol Ther 166:30–39
Newick K, Moon EK, Albelda SM (2016) Chimeric antigen receptor T-cell therapy for solid tumors. Mole Ther–Oncolytics 3:16006–16006
Tang N, Cheng C, Zhang X, Qiao M, Li N, Mu W, et al. (2020) TGF-beta inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight. 5:
Teng R, Zhao J, Zhao Y, Gao J, Li H, Zhou S et al (2019) Chimeric antigen receptor-modified T cells repressed solid tumors and their relapse in an established patient-derived colon carcinoma xenograft model. J Immunother 42:33–42
Ma S, Li X, Wang X, Cheng L, Li Z, Zhang C et al (2019) Current progress in CAR-T cell therapy for solid tumors. Int J Biol Sci 15:2548–2560
Ma L, Dichwalkar TM, Chang JY, Cossette B, Garafola D, Zhang AQ et al (2019) Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 365:162–168
Yamamoto TN, Kishton RJ, Restifo NP (2019) Developing neoantigen-targeted T cell–based treatments for solid tumors. Nat Med 25:1488–1499
Hintzen RQ, Lens SMA, Beckmann MP, Goodwin RG, Lynch DH, Van Lier RAW (1994) Characterization of the human CD27 ligand, a novel member of the TNF gene family. J Immunol 152:1762–1773
Jin L, Ge H, Long Y, Yang C, Chang Y, Mu L et al (2018) CD70, a novel target of CAR T-cell therapy for gliomas. Neuro Oncol 20:55–65
De Meulenaere A, Vermassen T, Aspeslagh S, Zwaenepoel K, Deron P, Duprez F et al (2016) CD70 expression and its correlation with clinicopathological variables in squamous cell carcinoma of the head and neck. Pathobiology 83:327–333
Zhang G, Jin G, Nie X, Mi R, Zhu G, Jia W et al (2014) Enhanced antitumor efficacy of an oncolytic herpes simplex virus expressing an endostatin-angiostatin fusion gene in human glioblastoma stem cell xenografts. PLoS ONE 9:e95872
Zhu G, Su W, Jin G, Xu F, Hao S, Guan F et al (2011) Glioma stem cells targeted by oncolytic virus carrying endostatin-angiostatin fusion gene and the expression of its exogenous gene in vitro. Brain Res 1390:59–69
Wang QJ, Yu Z, Hanada KI, Patel K, Kleiner D, Restifo NP et al (2017) Preclinical evaluation of chimeric antigen receptors targeting CD70-expressing cancers. Clin Cancer Res 23:2267–2276
Galea I, Bernardes-Silva M, Forse PA, van Rooijen N, Liblau RS, Perry VH (2007) An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J Exp Med 204:2023–2030
Klemm F, Maas R R, Bowman R L, Kornete M, Soukup K, Nassiri S, et al. (2020) Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 181: 1643–1660 e1617.
Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S et al (2018) Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 24:563–571
Tan A C, Ashley D M, Lopez G Y, Malinzak M, Friedman H S, Khasraw M (2020) Management of glioblastoma: state of the art and future directions. CA Cancer J Clin.
Zamarin D, Palese P (2012) Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiol 7:347–367
Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF (2005) Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol 174:4465–4469
Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G et al (2015) Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 28:415–428
Pikor LA, Bell JC, Diallo JS (2015) Oncolytic viruses: exploiting cancer’s deal with the devil. Trends Cancer 1:266–277
Son DS, Kabir SM, Dong Y, Lee E, Adunyah SE (2013) Characteristics of chemokine signatures elicited by EGF and TNF in ovarian cancer cells. J Inflamm (Lond) 10:25
Nagarsheth N, Wicha MS, Zou W (2017) Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol 17:559–572
Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA et al (2009) Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity 31:110–121
Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA et al (2009) The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther 20:1119–1132
Kanerva A, Nokisalmi P, Diaconu I, Koski A, Cerullo V, Liikanen I et al (2013) Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res 19:2734–2744
Schietinger A, Philip M, Liu RB, Schreiber K, Schreiber H (2010) Bystander killing of cancer requires the cooperation of CD4(+) and CD8(+) T cells during the effector phase. J Exp Med 207:2469–2477
Guedan S, Alemany R (2018) CAR-T cells and oncolytic viruses: joining forces to overcome the solid tumor challenge. Front Immunol 9:2460
Shultz LD, Ishikawa F, Greiner DL (2007) Humanized mice in translational biomedical research. Nat Rev Immunol 7:118–130
Zhu G D, Zhang Q, Zhang J W, Liu F S (2021) Targeting tumor-associated antigen: a promising CAR-T therapeutic strategy for glioblastoma treatment. Front Pharmacol. 12
Funding
This work was supported by grants from the Beijing Natural Science Foundation Program and Scientific Research Key Program of the Beijing Municipal Commission of Education (KZ202010025034), the Capital’s Funds for Health Improvement and Research (CFH, 2020–1-1071), the National Natural Science Foundation of China (No, 81672478), the Natural Science Foundation of Beijing Municipality (No. 7202020) and the Beijing Laboratory of Biomedical Materials Foundation.
Author information
Authors and Affiliations
Contributions
F.S.L and G.D.Z were responsible for design of the experiments, G.D.Z., J.W.Z and Q.Z performed the experiments, Q.Z., J.W.Z., F.S.L and G.S. J were responsible for acquisition of the data, G.D.Z., Q.Z. and X.D.S analyzed the data and prepared the manuscript, G.D.Z., J.W.Z., Q.Z., G.S.J., X.D.S. and F.S.L wrote the manuscript. All authors have read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval and consent to participate
The Experimental Animal Welfare and Ethics Committee of Beijing Tiantan Hospital affiliated to Capital Medical University and approved all the animal experiments. The use of tissue samples from patients with glioblastoma and the peripheral blood of healthy donors were approved by Ethics Committee of Beijing Tiantan Hospital affiliated to Capital Medical University.
Consent for publication
Not applicable. All authors read and approved the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, G., Zhang, J., Zhang, Q. et al. Enhancement of CD70-specific CAR T treatment by IFN-γ released from oHSV-1-infected glioblastoma. Cancer Immunol Immunother 71, 2433–2448 (2022). https://doi.org/10.1007/s00262-022-03172-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03172-x