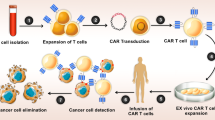
Abstract
Background
T cell with chimeric antigen receptors (CAR-T) has presented remarkable efficacy for blood cancer as an emerging immunotherapy. However, for solid tumors, the therapeutic efficacy is much impaired due to the lack of infiltration and persistence of CAR-T in tumor tissue. Thus, we constructed an interleukin-7-loaded oncolytic adenovirus and combined the use of oncolytic virus and CAR-T to improve the therapeutic outcome.
Methods
We constructed an interleukin-7-loaded oncolytic adenovirus (oAD-IL7) and a B7H3-targeted CAR-T and explored the efficacy of the single use of oAD-IL7, B7H3-CAR-T, or the combined therapy for glioblastoma in vitro and in vivo. The improved CAR-T anti-tumor efficacy was evaluated according to the proliferation, survival, persistence, exhaustion of T cells, and tumor regression.
Results
Constructed oAD-IL7 and B7H3-CAR-T presented moderate cytotoxicity during in vitro study, but failed to induce a thorough and persistent anti-tumor therapeutic efficacy in vivo. The combination of oAD-IL7 and B7H3-CAR-T in vitro resulted in enhanced T cell proliferation and reduced T cell apoptosis. The joint efficacy was further confirmed using tumor-bearing xenograft mice. During in vivo study, the mice treated with both oAD-IL7 and B7H3-CAR-T showed prolonged survival and reduced tumor burden. According to the ex vivo study, oAD-IL7 improved the proliferation and persistence of tumor-infiltrating B7H3-CAR-T, but failed to reverse the exhaustion.
Conclusions
Our results indicated that oAD-IL7 is a promising auxiliary therapy to improve the therapeutic efficacy of B7H3-CAR-T in glioblastoma by providing the activating signals for tumor-infiltrating T cells. Our results also lay the basis for the future clinical trials for the combination of IL7-loaded oncolytic adenovirus and CAR-T therapy for glioblastoma.





Similar content being viewed by others
Availability of data and material
All of the data relevant to this research are included in the article and supplementary files. Materials are available from corresponding author under reasonable request.
Abbreviations
- CAR-T:
-
Chimeric antigen receptor T cells
- DAMPs:
-
Damage-associated molecular patterns
- GBM:
-
Glioblastoma
- IL7:
-
Interleukin-7
- MDSC:
-
Myeloid-derived suppressor cells
- oAD:
-
Oncolytic adenovirus
- PAMPs:
-
Pathogen-associated molecular patterns
- scFv:
-
Single-chain variable fragment
References
Pagel JM, West HJ (2017) Chimeric antigen receptor (CAR) T-cell therapy. JAMA Oncol 11:1595
Calmes-Miller J (2018) FDA approves second CAR T-cell therapy. Cancer Discov 1:5–6
Li J, Li W, Huang K, Zhang Y, Kupfer G, Zhao Q (2018) Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol 1:22
Hegde PS, Chen DS (2020) Top 10 challenges in cancer immunotherapy. Immunity 1:17–35
Grosser R, Cherkassky L, Chintala N, Adusumilli PS (2019) Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell 5:471–482
Ma X, Shou P, Smith C, Chen Y, Du H, Sun C, Porterfield Kren N, Michaud D, Ahn S, Vincent B et al (2020) Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat Biotechnol 4:448–459
Harrington K, Freeman DJ, Kelly B, Harper J, Soria JC (2019) Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov 9:689–706
Hemminki O, Dos Santos JM, Hemminki A (2020) Oncolytic viruses for cancer immunotherapy. Journal of Hematol Oncol 1:84
Twumasi-Boateng K, Pettigrew JL, Kwok YYE, Bell JC, Nelson BH (2018) Oncolytic viruses as engineering platforms for combination immunotherapy. Nat Rev Cancer 7:419–432
Ge Y, Wang H, Ren J, Liu W, Chen L, Chen H, Ye J, Dai E, Ma C, Ju S et al (2020) Oncolytic vaccinia virus delivering tethered IL-12 enhances antitumor effects with improved safety. J Immunother Cancer 8(1):e000710
Nakao S, Arai Y, Tasaki M, Yamashita M, Murakami R, Kawase T, Amino N, Nakatake M, Kurosaki H, Mori M et al (2020) Intratumoral expression of IL7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aax7992
Perna SK, Pagliara D, Mahendravada A, Liu H, Brenner MK, Savoldo B, Dotti G (2014) Interleukin-7 mediates selective expansion of tumor-redirected cytotoxic T lymphocytes (CTLs) without enhancement of regulatory T-cell inhibition. Clin Cancer Res 1:131–139
Shum T, Omer B, Tashiro H, Kruse RL, Wagner DL, Parikh K, Yi Z, Sauer T, Liu D, Parihar R et al (2017) Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov 11:1238–1247
Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K (2018) IL7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol 4:346–351
Tang X, Zhao S, Zhang Y, Wang Y, Zhang Z, Yang M, Zhu Y, Zhang G, Guo G, Tong A et al (2019) B7–H3 as a novel CAR-T therapeutic target for glioblastoma. Mol Ther Oncol 14:279–287
Li X, Liu Y, Wen Z, Li C, Lu H, Tian M, Jin K, Sun L, Gao P, Yang E et al (2010) Potent anti-tumor effects of a dual specific oncolytic adenovirus expressing apoptin in vitro and in vivo. Mol Cancer 9:10
Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z (2006) Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev 211:81–92
Zhou J, Jin L, Wang F, Zhang Y, Liu B, Zhao T (2019) Chimeric antigen receptor T (CAR-T) cells expanded with IL7/IL-15 mediate superior antitumor effects. Protein Cell 10:764–769
Sidaway P (2018) CNS cancer: Oncolytic adenovirus effective in patients with glioblastoma. Nat Rev Clin Oncol 5:266
Qian C, Liu XY, Prieto J (2006) Therapy of cancer by cytokines mediated by gene therapy approach. Cell Res 2:182–188
Wing A, Fajardo CA, Posey AD, Shaw C, Da T, Young RM, Alemany R, June CH, Guedan S (2018) Improving CART-Cell Therapy of Solid Tumors with Oncolytic Virus-Driven Production of a Bispecific T-cell Engager. Cancer Immunol Res 5:605–616
Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, Rietberg SP, Linde MH, Xu P, Rota C et al (2019) CAR T cells targeting B7–H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res 8:2560–2574
Flem-Karlsen K, Fodstad Ø, Tan M, Nunes-Xavier CE (2018) B7–H3 in cancer—beyond immune regulation. Trends in Cancer 6:401–404
Zhang Z, Jiang C, Liu Z, Yang M, Tang X, Wang Y, Zheng M, Huang J, Zhong K, Zhao S et al (2020) B7-H3-Targeted CAR-T Cells Exhibit Potent Antitumor Effects on Hematologic and Solid Tumors. Mol Ther Oncol 17:180–189
Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J et al (2016) Regression of glioblastoma after chimeric antigen receptor T-cell therapy. New Engl J Med 26:2561–2569
Ahmed N, Brawley V, Hegde M, Bielamowicz K, Kalra M, Landi D, Robertson C, Gray TL, Diouf O, Wakefield A et al (2017) HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol 8:1094–1101
Cervera-Carrascon V, Siurala M, Santos JM, Havunen R, Tähtinen S, Karell P, Sorsa S, Kanerva A, Hemminki A (2018) TNFa and IL-2 armed adenoviruses enable complete responses by anti-PD-1 checkpoint blockade. Oncoimmunology 5:e1412902
Wang P, Li X, Wang J, Gao D, Li Y, Li H, Chu Y, Zhang Z, Liu H, Jiang G et al (2017) Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat Commun 1:1395
Fajardo CA, Guedan S, Rojas LA, Moreno R, Arias-Badia M, de Sostoa J, June CH, Alemany R (2017) Oncolytic adenoviral delivery of an EGFR-targeting T-cell engager improves antitumor efficacy. Can Res 8:2052–2063
Porter CE, Rosewell Shaw A, Jung Y, Yip T, Castro PD, Sandulache VC, Sikora A, Gottschalk S, Ittman MM, Brenner MK et al (2020) Oncolytic adenovirus armed with BiTE, cytokine, and checkpoint inhibitor enables CAR T cells to control the growth of heterogeneous tumors. Mol Ther 5:1251–1262
Ahmed M, Cheng M, Zhao Q, Goldgur Y, Cheal SM, Guo HF, Larson SM, Cheung NK (2015) Humanized affinity-matured monoclonal antibody 8H9 has potent antitumor activity and binds to FG loop of tumor antigen B7–H3. J Biol Chem 290(50):30018–30029. https://doi.org/10.1074/jbc.M115.679852
Acknowledgements
We thank Sheng Zenghua for the gift of H4 cell type. We also thank Cao Yi for the gift of glioblastoma tissue acquired from patients.
Funding
This work was supported by the National Natural Science Foundation of China (31471286 and 81772693), the National Major Scientific and Technological Special Project for Significant New Drugs Development (2015ZX09102010), the Postdoctoral Research Fund of Sichuan University (2018SCU12035), and Technology Innovation Project of Chengdu Science and Technology Bureau (No. 2019-YF05-00459-SN).
Author information
Authors and Affiliations
Contributions
LZ raised the idea. JH designed the experiment and completed it with MZ. ZZ contributed to the xenograft model construction. XT and YC drafted the manuscript. AP completed the statistics analysis, and AT and XP revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
All animal experiments followed a protocol approved by the Institutional Animal Care and Use Committee of Sichuan University.
Patient consent for publication
Not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the supplementary information.
Supplementary figure 1. Replication of oAD and oAD-IL7 in different GBM cell lines titrated by plaque assays
. The copies of both oAD and oAD-IL7 in U251 (A), U87 (B), A172 (C), T98G (D), and H4 (E) cell lines was titrated 24, 48, 72 hours after viral infection using plaque assay. Viral infection was performed at the MOI of 10. Supplementary figure 2. Cytotoxicity of oAD and oAD-IL7 in different GBM cell lines at different MOI. The cytotoxicity of oAD and oAD-IL7 for different GBM cell lines was measured at day 5 after infection at different MOI based on MTT analysis. (A-E) The 5 types of GBM cell lines. Supplementary figure 3. Construction of B7H3-CART based on multiple donors. The efficacy of CAR-T transduction (A) and activation (B) was monitored under T cells from different donators. Supplementary figure 4. The expression of B7-H3 and CXAR among glioblastoma according to The Cancer Genome Atlas (TCGA) Database. The prevalence (A) of B7H3 and CXAR and their relationship with patient survival (B) among GBM patients were analyzed based on the online data collected via TCGA database. Supplementary figure 5. Representative image of the cytotoxicity effect after serial coculturing B7H3-CART with oAD or oAD-IL7 infected U87 cell line. (A), (B), (C), (D) 2×105 U87 cells were infected by oAD or oAD-IL7 at the MOI of 10, and then cocultured with 1×106 untransduced T cells (UTD) or B7H3-CART for 3 days. The T cells were collected at day 4 for a repeated coculture assay, and the cytotoxicity of the second round of coculturing was measured at day 7 using MTT. Scale bar, 500μm. Supplementary figure 6. Plaque assay for the titration of oAD-IL7 in vivo. The infectious oAD-IL7 in the xenograft models was extracted by 3 consecutive freeze-thaw cycle, and quantified using plaque assay. The representative image is shown. Supplementary table 1. Nucleotide and amino acid sequence of B7H3-scfv. The B7H3-CART was constructed based on the B7H3-scfv. The amino and acid sequence of B7H3-scfv is shown in table. Red, heavy chain; Green, light chain; Yellow, linker.
Rights and permissions
About this article
Cite this article
Huang, J., Zheng, M., Zhang, Z. et al. Interleukin-7-loaded oncolytic adenovirus improves CAR-T cell therapy for glioblastoma. Cancer Immunol Immunother 70, 2453–2465 (2021). https://doi.org/10.1007/s00262-021-02856-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02856-0