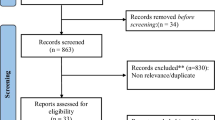
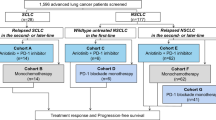
Abstract
Objectives
Programmed cell death-ligand 1 inhibitors plus chemotherapy (PD-L1 + Chemo) have achieved substantial progress in extensive-stage small-cell lung cancer (ES-SCLC). However, evidence about programmed cell death 1 inhibitors plus chemotherapy (PD-1 + Chemo) in SCLC is relatively lacking. Whether PD-1 inhibitors differ from PD-L1 inhibitors in their clinical outcomes remains controversial.
Materials and methods
We performed a meta-analysis to compare efficacy and safety of PD-L1 + Chemo vs PD-1 + Chemo in ES-SCLC by searching PubMed, Embase, the Cochrane Library, and major oncology conferences. We examined overall survival (OS) as the primary outcome. Secondary outcomes included progression-free survival (PFS), objective response rate (ORR), and treatment-related adverse events (AEs).
Results
We included four randomized trials (IMpower133, CASPIAN, KEYNOTE-604, and EA5161) with a total of 1553 patients. Direct comparison showed that PD-L1 + Chemo (PFS: hazard ratio [HR] 0.79; OS: HR 0.75) and PD-1 + Chemo (PFS: HR 0.72; OS: HR 0.77) significantly prolonged survival time compared with chemotherapy alone. But PD-L1 + Chemo (relative risk [RR]: 1.07) and PD-1 + Chemo (RR: 1.13) were not superior to chemotherapy alone in terms of ORR. Indirect comparison showed no significant difference in clinical efficacy between PD-L1 + Chemo and PD-1 + Chemo (OS: HR 0.99; PFS: HR 1.10; ORR: RR 0.95). We further stratified patients according to subgroups in terms of OS. In the subgroup of patients with brain metastasis, PD-L1 + Chemo tended to prolong OS (HR: 0.61, 0.28 to 1.32). There were no significant differences between PD-L1 + Chemo and PD-1 + Chemo regarding safety analyses. However, PD-L1 + Chemo exhibited a better safety profile in reducing the risk of treatment discontinuation due to AEs (RR: 0.43, 0.19 to 0.95) and pneumonia (pneumonia of any grade, RR: 0.59, 0.24 to 1.42; pneumonia of grade ≥ 3, RR: 0.37, 0.10 to 1.39).
Conclusions
PD-L1 + Chemo and PD-1 + Chemo provided a significant survival benefit relative to chemotherapy alone for ES-SCLC. The efficacy and safety of PD-L1 + Chemo and PD-1 + Chemo were similar based on current evidence.



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the published article.
Abbreviations
- AEs:
-
Adverse events
- BBB:
-
Blood-brain barrier
- Chemo:
-
Chemotherapy
- CI:
-
Confidence interval
- DCs:
-
Dendritic cells
- ECOG:
-
Eastern Cooperative Oncology Group
- ESMO:
-
European Society of Medical Oncology
- HRs:
-
Hazard ratios
- IC:
-
Immune cell
- ICIs:
-
Immune checkpoint inhibitors
- irAEs:
-
Immune-related adverse events
- NCCN:
-
National Comprehensive Cancer Network
- NSCLC:
-
Non-small-cell lung carcinoma
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD-1:
-
Programmed cell death 1
- PD-L1:
-
Programmed cell death-ligand 1
- PFS:
-
Progression-free survival
- RR:
-
Relative risk
- SE:
-
Standard error
- SCLC:
-
Small-cell lung cancer
- TC:
-
Tumor cell
- TMB:
-
Tumor mutational burden
References
Oronsky B, Reid T, Oronsky A, Carter C (2017) What’s new in SCLC? a review. Neoplasia (New York, NY) 19(10):842–847
Lally B, Urbanic J, Blackstock A, Miller A, Perry M (2007) Small cell lung cancer: have we made any progress over the last 25 years? Oncologist 12(9):1096–1104
Stahel R, Thatcher N, Früh M, Le Péchoux C, Postmus P, Sorensen J, Felip E (2011) 1st ESMO consensus conference in lung cancer; lugano 2010: small-cell lung cancer. Ann Oncol: Off J Eur Soc Med Oncol 22(9):1973–1980
Früh M, De Ruysscher D, Popat S, Crinò L, Peters S, Felip E (2013) Small-cell lung cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol : Off J Eur Soc Med Oncol 24:vi99–vi105
Kalemkerian G, Loo B, Akerley W, Attia A, Bassetti M, Boumber Y, Decker R, Dobelbower M, Dowlati A, Downey R et al (2018) NCCN guidelines insights: small cell lung cancer, version 2.2018. J Natl Compr Cancer Netw : JNCCN 16(10):1171–1182
Farago A, Keane F (2018) Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res 7(1):69–79
Liu S, Reck M, Mansfield A, Mok T, Scherpereel A, Reinmuth N, Garassino M, De Castro CJ, Califano R, Nishio M et al (2021) Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol : Off J Am Soc Clin Oncol 39(6):619–630
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair M, Özgüroğlu M, Ji J et al (2019) Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet (London, England) 394(10212):1929–1939
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair M, Powell S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092
Langer C, Gadgeel S, Borghaei H, Papadimitrakopoulou V, Patnaik A, Powell S, Gentzler R, Martins R, Stevenson J, Jalal S et al (2016) Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 17(11):1497–1508
Rudin C, Awad M, Navarro A, Gottfried M, Peters S, Csőszi T, Cheema P, Rodriguez-Abreu D, Wollner M, Yang J et al (2020) Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol : Off J Am Soc Clin Oncol 38(21):2369–2379
Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (CE) alone or in combination with nivolumab as frontline therapy for extensive-stage small cell lung cancer (ES-SCLC): ECOG-ACRIN EA5161 [https://ascopubs.org/doi/abs/https://doi.org/10.1200/JCO.2020.38.15_suppl.9000]
Bucher H, Guyatt G, Griffith L, Walter S (1997) The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 50(6):683–691
Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, Zhao Z, Zhao J, Chen S, Song J et al (2020) Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta-analysis. JAMA Oncol 6(3):375–384
Chen L, Han X (2015) Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Investig 125(9):3384–3391
Ando K, Manabe R, Kishino Y, Kusumoto S, Yamaoka T, Tanaka A, Ohmori T, Ohnishi T, Sagara H (2021) Comparative efficacy and safety of immunotherapeutic regimens with PD-1/PD-L1 inhibitors for previously untreated extensive-stage small cell lung cancer: a systematic review and network meta-analysis. Curr Oncol (Toronto, Ont) 28(2):1094–1113
Iams W, Porter J, Horn L (2020) Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol 17(5):300–312
Mayoux M, Roller A, Pulko V, Sammicheli S, Chen S, Sum E, Jost C, Fransen M, Buser R, Kowanetz M et al (2020) Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci Transl Med 12(534):eaav7431
Kowanetz M, Zou W, Gettinger S, Koeppen H, Kockx M, Schmid P, Kadel E, Wistuba I, Chaft J, Rizvi N et al (2018) Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1). Proc Natl Acad Sci USA 115(43):E10119–E10126
Xu C, Chen Y, Du X, Liu J, Huang C, Chen L, Zhou G, Li W, Mao Y, Hsu C et al (2018) Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ (Clinical research ed) 363:k4226
Khunger M, Rakshit S, Pasupuleti V, Hernandez A, Mazzone P, Stevenson J, Pennell N, Velcheti V (2017) Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest 152(2):271–281
Funding
This study was funded by grants 81903176 from the National Natural Science Funds of China; 2019A1515011596 from the Science and Technology Program of Guangdong Province. C2019110 from Medical Scientific Research Foundation of Guangdong Province. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
H.Y., P.C., and XY.C. contributed to data acquisition, data interpretation, and statistical analysis and drafting of the manuscript. C.C., XY.Z., and LN.H. contributed to data acquisition, data interpretation, and statistical analysis. YX.Z., SD.H., and B.Z. contributed to the study design, data acquisition, data interpretation, and statistical analysis. All the authors contributed to critical revision of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Précis: Our work confirmed that PD-L1+Chemo was not superior to PD-1+Chemo in terms of OS, PFS and ORR. However, PD-L1+Chemo tended to prolong OS for patients with brain metastases and exhibited a better safety profile relative to PD-1+Chemo.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, H., Chen, P., Cai, X. et al. Efficacy and safety of PD-L1 inhibitors versus PD-1 inhibitors in first-line treatment with chemotherapy for extensive-stage small-cell lung cancer. Cancer Immunol Immunother 71, 637–644 (2022). https://doi.org/10.1007/s00262-021-03017-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03017-z