Abstract
Background
Autologous monocyte-derived mRNA co-electroporated dendritic cells with mRNA encoding CD40 ligand (CD40L), CD70 and a constitutively activated TLR4 (caTLR4) (referred to as TriMixDC-MEL) have anti-tumor activity in advanced melanoma patients. We investigated the safety and activity of adjuvant TriMixDC-MEL in stage III/IV melanoma patients.
Materials and methods
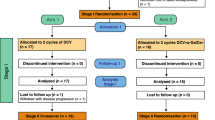
Forty-one patients were randomly assigned to treatment with TriMixDC-MEL (n = 21) and standard follow-up (n = 20). “Cross-over” was allowed at the time of non-salvageable recurrence. The primary endpoint was the percentage of patients alive and disease-free at 1-year. For a subset of patients, (formalin-fixed paraffin-embedded), tumor tissue samples were available for mRNA expression profiling and PD-L1 immunohistochemical staining.
Results
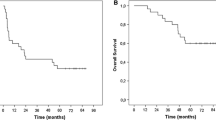
Baseline characteristics were well balanced. One-year after randomization, 71% of patients in the study arm were alive and free of disease compared to 35% in the control arm. After a median follow-up of 53 months (range 3–67), 23 patients experienced a non-salvageable melanoma recurrence (TriMixDC-Mel arm n = 9 and control arm n = 14).The median time to non-salvageable recurrence was superior in the TriMixDC-MEL arm (median 8 months (range 1–6) vs. not reached; log-rank p 0.044). TriMixDC-MEL-related adverse events (AE) consisted of transient local skin reactions, flu-like symptoms and post-infusion chills. No grade ≥ 3 AE’s occurred. The mRNA expression profiling revealed four genes (STAT2, TPSAB1, CD9 and CSF2) as potential predictive biomarkers.
Conclusion
TriMixDC-MEL id/iv as adjuvant therapy is tolerable and may improve the 1-year disease-free survival rate. Combination of optimized autologous monocyte-derived DC-formulations warrants further investigation in combination with currently approved adjuvant therapy options.


Similar content being viewed by others
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- %CV:
-
Percentage coefficient of variation
- AJCC:
-
American Joint Committee on Cancer
- AUC:
-
Area under the curve
- CD40-L:
-
CD40 ligand
- CLND:
-
Complete lymph node dissection
- DC:
-
Dendritic cells
- DMFS:
-
Distant metastasis free survival
- FFPE:
-
Formalin-fixed paraffin-embedded
- HKG:
-
Housekeeping genes
- id:
-
Intradermal
- iv:
-
Intravenous
- OS:
-
Overall survival
- RFS:
-
Recurrence-free survival
- TCGA:
-
The Cancer Genome Atlas
- TriMixDC-MEL:
-
Autologous monocyte-derived mRNA electroporated dendritic cells
- WHO-PS:
-
World Health Organization performance status
References
Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR et al (2017) IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 127(8):2930–2940. https://doi.org/10.1172/JCI91190
Balch CM, Gershenwald JE, Soong S et al (2010) Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol 28:2452–2459. https://doi.org/10.1200/JCO.2009.27.1627
Bol KF, Aarntzen EHJG, in’t Hout FEM et al (2016) Favorable overall survival in stage III melanoma patients after adjuvant dendritic cell vaccination. Oncoimmunology 5:1–8. https://doi.org/10.1080/2162402X.2015.1057673
Bonehill A, Tuyaerts S, Van Nuffel AMT et al (2008) Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol Ther 16:1170–1180. https://doi.org/10.1038/mt.2008.77
Bonehill A, Van Nuffel AMT, Corthals J et al (2009) Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients. Clin Cancer Res 15:3366–3375. https://doi.org/10.1158/1078-0432.CCR-08-2982
Byrd DR, Sondak VK, Soong S et al (2009) Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27:6199–6206. https://doi.org/10.1200/jco.2009.23.4799
Carrasco J, Van Pel A, Neyns B et al (2014) Vaccination of a melanoma patient with mature dendritic cells pulsed with MAGE-3 peptides triggers the activity of nonvaccine anti-tumor cells. J Immunol 180:3585–3593. https://doi.org/10.4049/jimmunol.180.5.3585
Daud AI, Wolchok JD, Robert C et al (2016) Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol 34:4102–4109. https://doi.org/10.1200/JCO.2016.67.2477
Eggermont AMM, Blank CU, Mandala M et al (2018) Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 378:1789–1801. https://doi.org/10.1056/NEJMoa1802357
Eggermont AMM, Chiarion-Sileni V, Grob J-J et al (2016) Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 375:1845–1855. https://doi.org/10.1056/NEJMoa1611299
Long GV, Hauschild A, Santinami M et al (2017) Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 377:1813–1823. https://doi.org/10.1056/nejmoa1708539
Markowicz S, Nowecki ZI, Rutkowski P et al (2012) Adjuvant vaccination with melanoma antigen-pulsed dendritic cells in stage III melanoma patients. Med Oncol 29:2966–2977. https://doi.org/10.1007/s12032-012-0168-1
Mocellin S, Lens MB, Pasquali S et al (2013) Interferon alpha for the adjuvant treatment of cutaneous melanoma. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd008955.pub2
Ollila DW (2006) Complete metastasectomy in patients with stage IV metastatic melanoma. Lancet Oncol 7:919–924. https://doi.org/10.1016/S1470-2045(06)70938-X
Romano E, Scordo M, Dusza SW et al (2010) Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J Clin Oncol 28:3042–3047. https://doi.org/10.1200/JCO.2009.26.2063
Van Nuffel AMT, Benteyn D, Wilgenhof S et al (2012) Dendritic cells loaded with mRNA encoding full-length tumor antigens prime CD4 + and CD8 + T cells in melanoma patients. Mol Ther 20:1063–1074. https://doi.org/10.1038/mt.2012.11
Van Nuffel AMT, Benteyn D, Wilgenhof S et al (2012) Intravenous and intradermal TriMix-dendritic cell therapy results in a broad T-cell response and durable tumor response in a chemorefractory stage IV-M1c melanoma patient. Cancer Immunol Immunother 61:1033–1043. https://doi.org/10.1007/s00262-011-1176-2
Weber J, Mandala M, Del Vecchio M et al (2017) Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 377:1824–1835. https://doi.org/10.1056/NEJMoa1709030
Wilgenhof S, Corthals J, Heirman C et al (2016) Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol 34:1330–1338. https://doi.org/10.1200/JCO.2015.63.4121
Wilgenhof S, Corthals J, Van Nuffel AMT et al (2015) Long-term clinical outcome of melanoma patients treated with messenger RNA-electroporated dendritic cell therapy following complete resection of metastases. Cancer Immunol Immunother 64:381–388. https://doi.org/10.1007/s00262-014-1642-8
Wilgenhof S, Van Nuffel AMT, Benteyn D et al (2013) A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol Off J Eur Soc Med Oncol 24:2686–2693. https://doi.org/10.1093/annonc/mdt245
Wilgenhof S, Van Nuffel AMT, Corthals J et al (2011) Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. J Immunother 34:448–456. https://doi.org/10.1097/CJI.0b013e31821dcb31
Acknowledgements
We thank the patients who participated in the study, their families and caregivers. We would also like to thank Katrien Van den Bossche for assisting us with the data management.
Funding
This study was funded by Rijksinstituut voor Ziekte- en Invaliditeitsverzekering Belgium.
Author information
Authors and Affiliations
Contributions
YJ contributed to first draft, patient treatment, statistical analyses. VK contributed to patient treatment, revision of draft. JC contributed to manufacturing of study drug, revision of draft. KS contributed to analyses of samples, revision of draft, statistical analyses. TS contributed to patient treatment, revision of draft, statistical analyses. P-JVD contributed to analyses of samples, revision of draft, statistical analyses. CH contributed to manufacturing of study drug, revision of draft. LB contributed to patient treatment, revision of draft. MK contributed to analyses of samples, revision of draft, statistical analyses. KT contributed to concept of study, manufacturing of study drug, revision of draft, statistical analyses. BN contributed to concept of study, patient treatment, revision of draft, statistical analyses.
Corresponding author
Ethics declarations
Conflict of interest
Yanina Jansen received travel grant support from BMS, MSD, Pfizer. Vibeke Kruse provided consultation, attended advisory boards, and/or provided lectures for: Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Amgen and Sanofi. My institution received honoraria for my contribution. Jurgen Corthals has no conflict of interest. Kelly Schats: KS is an employee of HistoGeneX NV which performs immunohistochemical and molecular testing for pharmaceutical companies as part of (pre-) clinical studies that evaluate new anticancer drugs. There is no other relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Pieter-Jan Van Dam4: PJ Van Dam is an employee of HistoGeneX NV which performs immunohistochemical and molecular testing for pharmaceutical companies as part of (pre-) clinical studies that evaluate new anticancer drugs. There is no other relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Teofila Seremet received congresses and advisory fees from Novartis and one preceptorship MSD. Carlo Heirman: CH is an inventor on patents related to TriMix and is employee of eTheRNA ImmunoTherapies and may therefore have a financial interest in the development of TriMix technologies. Lieve Brochez: Advisory Board Incyte 6/2017, Value-based health care project sponsored by Amgen. Mark Kockx: Mark Kockx is the CEO of HistoGeneX NV which performs immunohistochemical and molecular testing for pharmaceutical companies as part of (pre-) clinical studies that evaluate new anticancer drugs. There is no other relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Kris Thielemans: KT is an inventor on patents related to TriMix and DC technology and is consultant for eTheRNA ImmunoTherapies and may therefore has a financial interest in the development of TriMix technologies. Bart Neyns: Honoraria-Bristol-Myers Squibb; Merck Sharp & Dohme; Novartis; Roche. Consulting or Advisory Role-Bristol-Myers Squibb; Merck Sharp & Dohme; Novartis; Roche. Speakers’ Bureau-Novartis. Travel, Accommodations, Expenses-Amgen; Bristol-Myers Squibb; Merck Sharp & Dohme; Novartis; Roche.
Ethical approval and consent to participate
This trial was approved by the Institutional Ethics Committee of the UZ Brussel (ClinicalTrials.gov identifier: NCT01676779). All patients provided signed informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jansen, Y., Kruse, V., Corthals, J. et al. A randomized controlled phase II clinical trial on mRNA electroporated autologous monocyte-derived dendritic cells (TriMixDC-MEL) as adjuvant treatment for stage III/IV melanoma patients who are disease-free following the resection of macrometastases. Cancer Immunol Immunother 69, 2589–2598 (2020). https://doi.org/10.1007/s00262-020-02618-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02618-4