Abstract
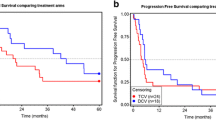
Dendritic cells may be successfully used to induce in vivo-specific anti-tumor responses when combined with the appropriate antigen in the appropriate context. The purpose of this study was to evaluate efficacy of peptide-loaded DC vaccine in high-risk stage III melanoma patients after lymph node dissection (LND). HLA-A2+, -A1+, or -A3+ melanoma patients (N = 22), stage III, N1b-N3, received 5–16 (median: 11) DC vaccines loaded with MHC class-I-restricted melanoma peptides respective to the patient’s haplotype, and with autologous tumor lysate, if available. Vaccinated patients were matched to unvaccinated stage III controls (22 of 869) by sex, number of metastatic lymph nodes, extracapsular involvement, LND type, Breslow stage, and ulceration. Vaccination elicited cutaneous delayed-type hypersensitivity (DTH) or/and IFN-γ-producing CD8+ cell response to melanoma peptides in 15 of 22 patients. Three-year overall survival (OS) rate was 68.2% in the vaccinated group versus 25.7% in the control group, P value accounting for matching: 0.0290. In a Cox regression model, hazard ratio (HR) for death of vaccinated patients was 0.31 [95% confidence interval (CI): 0.10–0.94]. The corresponding values for 3-year disease-free survival rate were 40.9 versus 14.5%, P = 0.1083; HR of recurrence for vaccinated, 0.46 (95% CI: 0.18–1.22). There was no grade >1 toxicity. The DC/peptide vaccine was well tolerated and elicited immune responses to melanoma antigens. Vaccinated patients had significantly longer OS after LND than the matched controls, but a significant improvement in the primary endpoint DFS was not achieved.





Similar content being viewed by others
References
Mehta-Damani A, Markowicz S, Engleman EG. Generation of antigen-specific CD8+ CTLs from naive precursors. J Immunol. 1994;153:996–1003.
Mehta-Damani A, Markowicz S, Engleman EG. Generation of antigen-specific CD4+ T cell lines from naive precursors. Eur J Immunol. 1995;25:1206–11.
Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–8.
Timmerman JM, Czerwinski DK, Davis TA, et al. Idiotype pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517–26.
Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22.
Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–32.
Banchereau J, Palucka AK, Dhodapkar M, et al. Immune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–8.
O’Rourke MG, Johnson M, Lanagan C, et al. Durable complete clinical responses in a phase I/II trial using an autologous melanoma cell/dendritic cell vaccine. Cancer Immunol Immunother. 2003;52:387–95.
O’Rourke MG, Johnson MK, Lanagan CM, et al. Dendritic cell immunotherapy for stage IV melanoma. Melanoma Res. 2007;17:316–22.
Schadendorf D, Ugurel S, Schuler-Thurner B, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol. 2006;17:563–70.
Engell-Noerregaard L, Hansen TH, Andersen MH, Thor Straten P, Svane IM. Review of clinical studies on dendritic cell-based vaccination of patients with malignant melanoma: assessment of correlation between clinical response and vaccine parameters. Cancer Immunol Immunother. 2009;58:1–14.
Schadendorf D, Algarra SM, Bastholt L, et al. Immunotherapy of distant metastatic disease. Ann Oncol. 2009;20(Suppl 6):vi41–50.
Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15.
Koos D, Josephs SF, Alexandrescu DT, et al. Tumor vaccines in 2010: need for integration. Cell Immunol. 2010;263:138–47.
Lesterhuis WJ, Aarntzen EH, De Vries IJ, et al. Dendritic cell vaccines in melanoma: from promise to proof? Crit Rev Oncol Hematol. 2008;66:118–34.
Letsch A, Keilholz U, Fluck M, et al. Peptide vaccination after repeated resection of metastases can induce a prolonged relapse-free interval in melanoma patients. Int J Cancer. 2005;114:936–41.
Tuettenberg A, Becker C, Huter E, Knop J, Enk AH, Jonuleit H. Induction of strong and persistent MelanA/MART-1-specific immune responses by adjuvant dendritic cell-based vaccination of stage II melanoma patients. Int J Cancer. 2006;118:2617–27.
Testori A, Rutkowski P, Marsden J, et al. Surgery and radiotherapy in the treatment of cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi22–9.
Mortarini R, Anichini A, Di Nicola M, et al. Autologous dendritic cells derived from CD34+ progenitors and from monocytes are not functionally equivalent antigen-presenting cells in the induction of MelanA/Mart-127-35-specific CTLs from peripheral blood lymphocytes of melanoma patients with low frequency of CTL precursors. Cancer Res. 1997;57:5534–41.
Nowecki ZI, Rutkowski P, Michej W. The survival benefit to patients with positive sentinel node melanoma after completion lymph node dissection may be limited to the subgroup with a primary lesion Breslow thickness greater than 1.0 and less than or equal to 4 mm (pT2-pT3). Ann Surg Oncol. 2008;15:2223–34.
Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. New York: Springer; 1997. p. 282–6.
Thompson LW, Garbee CF, Hibbitts S, et al. Competition among peptides in melanoma vaccines for binding to MHC molecules. J Immunother. 2004;27:425–31.
Slingluff CL Jr, Petroni GR, Chianese-Bullock KA, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13:6386–95.
Tsai V, Southwood S, Sidney J, et al. Identification of subdominant CTL epitopes of the gp100 melanoma-associated tumor antigen by primary in vitro immunization with peptide-pulsed dendritic cells. J Immunol. 1997;158:1796–802.
Markowicz S, Engleman EG. Granulocyte-macrophage colony-stimulating factor promotes differentiation and survival of human peripheral blood dendritic cells in vitro. J Clin Invest. 1990;85:955–61.
Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–61.
Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18:226–32.
Eggermont AM. Immunostimulation versus immunosuppression after multiple vaccinations: the woes of therapeutic vaccine development. Clin Cancer Res. 2009;15:6745–7.
Faries MB, Hsueh EC, Ye X, Hoban M, Morton DL. Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogeneic whole-cell melanoma vaccine. Clin Cancer Res. 2009;15:7029–35.
Slingluff CL Jr, Petroni GR, Olson WC, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15:7036–44.
Gunturu KS, Meehan KR, Mackenzie TA, et al. Cytokine working group study of lymphodepleting chemotherapy, interleukin-2, and granulocyte-macrophage colony-stimulating factor in patients with metastatic melanoma: clinical outcomes and peripheral-blood cell recovery. J Clin Oncol. 2010;28:1196–202.
Santiago-Schwarz F, Belilos E, Diamond B, Carsons SE. TNF in combination with GM-CSF enhances the differentiation of neonatal cord blood stem cells into dendritic cells and macrophages. J Leuk Biol. 1992;52:274–81.
Reid CDL, Stackpoole A, Meager A, Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992;149:2681–8.
Young JW, Szabolcs P, Moore MAS. Identification of dendritic cell colony-forming units among normal human CD34+ bone marrow progenitors that are expanded by c-kit-ligand and yield pure dendritic cell colonies in the presence of granulocyte/macrophage colony-stimulating factor and tumor necrosis factor α. J Exp Med. 1995;182:1111–9.
Siena S, Di Nicola M, Bregni M, et al. Massive ex vivo generation of functional dendritic cells from mobilized CD34+ blood progenitors for anticancer therapy. Exp Hematol. 1995;23:1463–71.
Markowicz S, Walewski J, Kawecki A. Isolation and characteristic of dendritic cell progenitors from the bone marrow of the Hodgkin’s disease patients. In: Banchereau J, Schmitt D, editors. Dendritic cells in fundamental and clinical immunology, vol. 2. New York: Plenum Press; 1995. p. 553–5.
Bernhard H, Disks ML, Heimfeld S, Hand S, Gralow JR, Cheever MA. Generation of immunostimulatory dendritic cells from human CD34+ hematopoietic progenitor cells of the bone marrow and peripheral blood. Cancer Res. 1995;55:1099–104.
Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18.
Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–61.
Hayashi Y, Hoon DS, Park MS, Terasaki PI, Foshag LJ, Morton DL. Induction of CD4+ cytotoxic T cells by sensitization with allogeneic melanomas bearing shared or cross-reactive HLA-A. Cell Immunol. 1992;139:411–25.
Robbins PF, el-Gamil M, Li YF, et al. Cloning of a new gene encoding an antigen recognized by melanoma-specific HLA-A24-restricted tumor-infiltrating lymphocytes. J Immunol. 1995;154:5944–50.
Darrow TL, Abdel-Wahab Z, Quinn-Allen MA, Seigler HF. Recognition and lysis of human melanoma by a CD3+, CD4+, CD8− T-cell clone restricted by HLA-A2. Cell Immunol. 1996;172:52–9.
Nishimura MI, Avichezer D, Custer MC, et al. MHC class I-restricted recognition of a melanoma antigen by a human CD4+ tumor infiltrating lymphocyte. Cancer Res. 1999;59:6230–8.
Voelkl S, Moore TV, Rehli M, Nishimura MI, Mackensen A, Fischer K. Characterization of MHC class-I restricted TCRalphabeta+ CD4− CD8− double negative T cells recognizing the gp100 antigen from a melanoma patient after gp100 vaccination. Cancer Immunol Immunother. 2009;58:709–18.
Sosman JA, Unger JM, Liu PY, et al. Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: impact of HLA class I antigen expression on outcome. J Clin Oncol. 2002;8:2067–75.
Wolchok JD, Weber JS, Hamid O, et al. Ipilimumab efficacy and safety in patients with advanced melanoma: a retrospective analysis of HLA subtype from four trials. Cancer Immun. 2010;10:9–14.
Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Acknowledgments
We thank Magdalena Rosinska for statistical assistance.
Conflict of interest
All authors declare that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Markowicz, S., Nowecki, Z.I., Rutkowski, P. et al. Adjuvant vaccination with melanoma antigen-pulsed dendritic cells in stage III melanoma patients. Med Oncol 29, 2966–2977 (2012). https://doi.org/10.1007/s12032-012-0168-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-012-0168-1