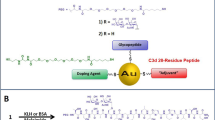
Abstract
The construction of a tumor-associated carbohydrate antigen-zwitterionic polysaccharide conjugate, Thomsen-nouveau-polysaccharide A1 (Tn-PS A1, where Tn = d-GalpNAc), has led to the development of a carbohydrate binding monoclonal antibody named Kt-IgM-8. Kt-IgM-8 was produced via hybridoma from Tn-PS A1 hyperimmunized Jackson Laboratory C57BL/6 mice, splenocytes and the murine myeloma cell line Sp2/0Ag14 with subsequent cloning on methyl cellulose semi-solid media. This in-house generated monoclonal antibody negates binding influenced from peptides, proteins, and lipids and preferentially binds monovalent Tn antigen as noted by ELISA, FACS, and glycan array technologies. Kt-IgM-8 demonstrated in vitro and in vivo tumor killing against the Michigan Cancer Foundation breast cell line 7 (MCF-7). In vitro tumor killing was observed using an LDH assay that measured antibody-induced complement-dependent cytotoxicity and these results were validated in an in vivo passive immunotherapy approach using an MCF-7 cell line-derived xenograft model. Kt-IgM-8 is effective in killing tumor cells at 30% cytotoxicity, and furthermore, it demonstrated approximately 40% reduction in tumor growth in the MCF-7 model.






Similar content being viewed by others
Abbreviations
- 10-DMEM:
-
10% FCS-DMEM
- 20-DMEM:
-
20% FCS-DMEM
- ATCC:
-
American-type culture collection
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- BD:
-
Becton Dickinson
- CDC:
-
Complement-dependent cytotoxicity
- Cy3:
-
Cyanine 3
- DMB:
-
1,2-Diamino-4,5-methylenedioxybenzene
- FDA:
-
Food and Drug Administration
- GD2:
-
Ganglioside disialic acid 2
- HCT-116:
-
Human colorectal carcinoma
- KLH:
-
Keyhole limpet hemocyanin
- MCF-7:
-
Michigan Cancer Foundation breast cell line 7
- mIgG:
-
Monoclonal IgG
- mIgM:
-
Monoclonal IgM
- MS:
-
Mass spectrometry
- NCI:
-
National Cancer Institute
- NCTC:
-
National collection of type cultures
- NMR:
-
Nuclear magnetic resonance spectroscopy
- pIgG:
-
Polyclonal IgG
- pIgM:
-
Polyclonal IgM
- PNPP:
-
Para-nitrophenylphosphate
- PS A1:
-
Polysaccharide A1
- STn:
-
Sialyl Thomsen-nouveau
- TACA:
-
Tumor-associated carbohydrate antigen
- TF:
-
Thomsen–Friedenreich
- Tn:
-
Thomsen-nouveau
- VVL:
-
Vicia villosa lectin
- ZPS:
-
Zwitterionic polysaccharide
References
Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM (2009) The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 15(17):5323–5337. https://doi.org/10.1158/1078-0432.ccr-09-0737
O’Cearbhaill RE, Ragupathi G, Zhu J, Wan Q, Mironov S, Yang G, Spassova MK, Iasonos A, Kravetz S, Ouerfelli O, Spriggs DR, Danishefsky SJ, Sabbatini PJ (2016) A phase I study of unimolecular pentavalent (Globo-H-GM2-STn-TF-Tn) immunization of patients with epithelial ovarian, fallopian tube, or peritoneal cancer in first remission. Cancers (Basel). https://doi.org/10.3390/cancers8040046
Laubreton D, Bay S, Sedlik C, Artaud C, Ganneau C, Deriaud E, Viel S, Puaux AL, Amigorena S, Gerard C, Lo-Man R, Leclerc C (2016) The fully synthetic MAG-Tn3 therapeutic vaccine containing the tetanus toxoid-derived TT830-844 universal epitope provides anti-tumor immunity. Cancer Immunol Immunother 65(3):315–325. https://doi.org/10.1007/s00262-016-1802-0
Scheid E, Major P, Bergeron A, Finn OJ, Salter RD, Eady R, Yassine-Diab B, Favre D, Peretz Y, Landry C, Hotte S, Mukherjee SD, Dekaban GA, Fink C, Foster PJ, Gaudet J, Gariepy J, Sekaly RP, Lacombe L, Fradet Y, Foley R (2016) Tn-MUC1 DC vaccination of rhesus macaques and a phase I/II trial in patients with nonmetastatic castrate-resistant prostate cancer. Cancer Immunol Res 4(10):881–892. https://doi.org/10.1158/2326-6066.cir-15-0189
Gilewski T, Ragupathi G, Bhuta S, Williams LJ, Musselli C, Zhang XF, Bornmann WG, Spassova M, Bencsath KP, Panageas KS, Chin J, Hudis CA, Norton L, Houghton AN, Livingston PO, Danishefsky SJ (2001) Immunization of metastatic breast cancer patients with a fully synthetic Globo H conjugate: a phase I trial. Proc Natl Acad Sci USA 98(6):3270–3275. https://doi.org/10.1073/pnas.051626298
Son HY, Apostolopoulos V, Kim CW (2016) T/Tn immunotherapy avoiding immune deviation. Int J Immunopathol Pharmacol 29(4):812–817. https://doi.org/10.1177/0394632016674018
Dhillon S (2015) Dinutuximab: first global approval. Drugs 75(8):923–927. https://doi.org/10.1007/s40265-015-0399-5
Aggarwal RS (2014) What’s fueling the biotech engine—2012 to 2013. Nat Biotechnol 32(1):32–39. https://doi.org/10.1038/nbt.2794
Weiner GJ (2015) Building better monoclonal antibody-based therapeutics. Nat Rev Cancer 15(6):361–370. https://doi.org/10.1038/nrc3930
Buskas T, Li Y, Boons GJ (2004) The immunogenicity of the tumor-associated antigen Lewis(Y) may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chem Eur J 10(14):3517–3524. https://doi.org/10.1002/chem.200400074
Kudryashov V, Glunz PW, Williams LJ, Hintermann S, Danishefsky SJ, Lloyd KO (2001) Toward optimized carbohydrate-based anticancer vaccines: Epitope clustering, carrier structure, and adjuvant all influence antibody responses to Lewis(Y) conjugates in mice. Proc Natl Acad Sci USA 98(6):3264–3269. https://doi.org/10.1073/pnas.051623598
Kagan E, Ragupathi G, Yi SS, Reis CA, Gildersleeve J, Kahne D, Clausen H, Danishefsky SJ, Livingston PO (2005) Comparison of antigen constructs and carrier molecules for augmenting the immunogenicity of the monosaccharide epithelial cancer antigen Tn. Cancer Immunol Immunother 54(5):424–430. https://doi.org/10.1007/s00262-004-0584-y
Ibrahim NK, Murray JL (2003) Clinical development of the STn-KLH vaccine (THERATOPE®). Clin Breast Cancer 3(Suppl 4):S139–S143. https://doi.org/10.3816/CBC.2003.s.003
Holmberg LA, Sandmaier BM (2001) Theratope vaccine (STn-KLH). Expert Opin Biol Ther 1(5):881–891. https://doi.org/10.1517/14712598.1.5.881
De Silva RA, Wang Q, Chidley T, Appulage DK, Andreana PR (2009) Immunological response from an entirely carbohydrate antigen: design of synthetic vaccines based on Tn-PS A1 conjugates. J Am Chem Soc 131(28):9622–9623. https://doi.org/10.1021/ja902607a
Kalia J, Raines RT (2008) Hydrolytic stability of hydrazones and oximes. Angew Chem Int Ed Engl 47(39):7523–7526. https://doi.org/10.1002/anie.200802651
De Silva RA, Appulage DK, Pietraszkiewicz H, Bobbitt KR, Media J, Shaw J, Valeriote FA, Andreana PR (2012) The entirely carbohydrate immunogen Tn-PS A1 induces a cancer cell selective immune response and cytokine IL-17. Cancer Immunol Immunother 61(4):581–585. https://doi.org/10.1007/s00262-012-1205-9
Shi M, Kleski KA, Trabbic KR, Bourgault JP, Andreana PR (2016) Sialyl-Tn polysaccharide A1 as an entirely carbohydrate immunogen: synthesis and immunological evaluation. J Am Chem Soc 138(43):14264–14272. https://doi.org/10.1021/jacs.6b05675
Irie RF, Ollila DW, O’Day S, Morton DL (2004) Phase I pilot clinical trial of human IgM monoclonal antibody to ganglioside GM3 in patients with metastatic melanoma. Cancer Immunol Immunother 53(2):110–117. https://doi.org/10.1007/s00262-003-0436-1
Liedtke M, Twist CJ, Medeiros BC, Gotlib JR, Berube C, Bieber MM, Bhat NM, Teng NN, Coutre SE (2012) Phase I trial of a novel human monoclonal antibody mAb216 in patients with relapsed or refractory B-cell acute lymphoblastic leukemia. Haematologica 97(1):30–37. https://doi.org/10.3324/haematol.2011.045997
Melis JP, Strumane K, Ruuls SR, Beurskens FJ, Schuurman J, Parren PW (2015) Complement in therapy and disease: regulating the complement system with antibody-based therapeutics. Mol Immunol 67(2 Pt A):117–130. https://doi.org/10.1016/j.molimm.2015.01.028
Brandlein S, Pohle T, Ruoff N, Wozniak E, Muller-Hermelink HK, Vollmers HP (2003) Natural IgM antibodies and immunosurveillance mechanisms against epithelial cancer cells in humans. Cancer Res 63(22):7995–8005
Imai M, Landen C, Ohta R, Cheung NK, Tomlinson S (2005) Complement-mediated mechanisms in anti-GD2 monoclonal antibody therapy of murine metastatic cancer. Cancer Res 65(22):10562–10568. https://doi.org/10.1158/0008-5472.can-05-1894
Czajkowsky DM, Shao Z (2009) The human IgM pentamer is a mushroom-shaped molecule with a flexural bias. Proc Natl Acad Sci USA 106(35):14960–14965. https://doi.org/10.1073/pnas.0903805106
Ehrenstein MR, Notley CA (2010) The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol 10(11):778–786. https://doi.org/10.1038/nri2849
Manimala JC, Roach TA, Li Z, Gildersleeve JC (2007) High-throughput carbohydrate microarray profiling of 27 antibodies demonstrates widespread specificity problems. Glycobiology 17(8):17c–23c. https://doi.org/10.1093/glycob/cwm047
Karsten U, Diotel C, Klich G, Paulsen H, Goletz S, Muller S, Hanisch FG (1998) Enhanced binding of antibodies to the DTR motif of MUC1 tandem repeat peptide is mediated by site-specific glycosylation. Cancer Res 58(12):2541–2549
Karsten U, Serttas N, Paulsen H, Danielczyk A, Goletz S (2004) Binding patterns of DTR-specific antibodies reveal a glycosylation-conditioned tumor-specific epitope of the epithelial mucin (MUC1). Glycobiology 14(8):681–692. https://doi.org/10.1093/glycob/cwh090
Ohyabu N, Kakiya K, Yokoi Y, Hinou H, Nishimura S (2016) Convergent solid-phase synthesis of macromolecular MUC1 models truly mimicking serum glycoprotein biomarkers of interstitial lung diseases. J Am Chem Soc 138(27):8392–8395. https://doi.org/10.1021/jacs.6b04973
Rangappa S, Artigas G, Miyoshi R, Yokoi Y, Hayakawa S, Garcia-Martin F, Hinou H, Nishimura S-I (2016) Effects of the multiple O-glycosylation states on antibody recognition of the immunodominant motif in MUC1 extracellular tandem repeats. MedChemComm 7(6):1102–1122. https://doi.org/10.1039/C6MD00100A
Li Q, Anver MR, Butcher DO, Gildersleeve JC (2009) Resolving conflicting data on expression of the Tn antigen and implications for clinical trials with cancer vaccines. Mol Cancer Ther 8(4):971–979. https://doi.org/10.1158/1535-7163.mct-08-0934
Oyelaran O, Li Q, Farnsworth D, Gildersleeve JC (2009) Microarrays with varying carbohydrate density reveal distinct subpopulations of serum antibodies. J Proteome Res 8(7):3529–3538. https://doi.org/10.1021/pr9002245
Jegerlehner A, Wiesel M, Dietmeier K, Zabel F, Gatto D, Saudan P, Bachmann MF (2010) Carrier induced epitopic suppression of antibody responses induced by virus-like particles is a dynamic phenomenon caused by carrier-specific antibodies. Vaccine 28(33):5503–5512. https://doi.org/10.1016/j.vaccine.2010.02.103
Schutze MP, Leclerc C, Jolivet M, Audibert F, Chedid L (1985) Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J Immunol 135(4):2319–2322
Andrew SM, Titus JA, Coico R, Amin A (2001) Purification of immunoglobulin M and immunoglobulin D. Curr Protoc Immunol. https://doi.org/10.1002/0471142735.im0209s21 (Chap. 2:Unit 2.9)
Prendergast JM, Galvao da Silva AP, Eavarone DA, Ghaderi D, Zhang M, Brady D, Wicks J, DeSander J, Behrens J, Rueda BR (2017) Novel anti-sialyl-Tn monoclonal antibodies and antibody-drug conjugates demonstrate tumor specificity and anti-tumor activity. MAbs 9(4):615–627. https://doi.org/10.1080/19420862.2017.1290752
Kudryashov V, Ragupathi G, Kim IJ, Breimer ME, Danishefsky SJ, Livingston PO, Lloyd KO (1998) Characterization of a mouse monoclonal IgG3 antibody to the tumor-associated Globo H structure produced by immunization with a synthetic glycoconjugate. Glycoconj J 15(3):243–249. https://doi.org/10.1023/A:1006992911709
Heimburg-Molinaro J, Rittenhouse-Olson K (2009) Development and characterization of antibodies to carbohydrate antigens. Methods Mol Biol 534:341–357. https://doi.org/10.1007/978-1-59745-022-5_24
Freire T, Bay S, von Mensdorff-Pouilly S, Osinaga E (2005) Molecular basis of incomplete O-glycan synthesis in MCF-7 breast cancer cells: putative role of MUC6 in Tn antigen expression. Cancer Res 65(17):7880–7887. https://doi.org/10.1158/0008-5472.can-04-3746
Oura F, Yajima Y, Nakata M, Taniue K, Akiyama T, Nakada H, Yamamoto K, Fujita-Yamaguchi Y (2015) Susceptibility to proteases of anti-Tn-antigen MLS128 binding glycoproteins expressed in human colon cancer cells. Biosci Trends 9(1):49–55. https://doi.org/10.5582/bst.2014.01127
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1):11–30. https://doi.org/10.3322/caac.21166
Koo GC, Hasan A, O’Reilly RJ (2009) Use of humanized severe combined immunodeficient mice for human vaccine development. Expert Rev Vaccines 8(1):113–120. https://doi.org/10.1586/14760584.8.1.113
Samraj AN, Pearce OM, Laubli H, Crittenden AN, Bergfeld AK, Banda K, Gregg CJ, Bingman AE, Secrest P, Diaz SL, Varki NM, Varki A (2015) A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci USA 112(2):542–547. https://doi.org/10.1073/pnas.1417508112
Welinder C, Baldetorp B, Borrebaeck C, Fredlund BM, Jansson B (2011) A new murine IgG1 anti-Tn monoclonal antibody with in vivo anti-tumor activity. Glycobiology 21(8):1097–1107. https://doi.org/10.1093/glycob/cwr048
Ando H, Matsushita T, Wakitani M, Sato T, Kodama-Nishida S, Shibata K, Shitara K, Ohta S (2008) Mouse-human chimeric anti-Tn IgG1 induced anti-tumor activity against Jurkat cells in vitro and in vivo. Biol Pharm Bull 31(9):1739–1744. https://doi.org/10.1248/bpb.31.1739
Nakada H, Inoue M, Numata Y, Tanaka N, Funakoshi I, Fukui S, Mellors A, Yamashina I (1993) Epitopic structure of Tn glycophorin a for an anti-Tn antibody (MLS 128). Proc Natl Acad Sci USA 90(6):2495–2499. https://doi.org/10.1073/pnas.90.6.2495
Acknowledgements
We are grateful to Fred Valeriote, Joe Media, and Halina Pietrazkiewicz (Henry Ford Health System) for donating human tumor cell lines as well as insightful discussions regarding SCID mice models. In addition, we would like to thank Katherine Goans (DLAR, University of Toledo) for assistance with surgical xenograft procedures on SCID mice.
Funding
The research was funded by the National Institutes of Health/National Cancer Institute under Grant 1R01 CA156661 and in part by the National Cancer Institute of the National Institutes of Health Small Business Innovation Research under Grants 1R43CA186326-01A1, HHSN261200700063C and HHSN261200900034C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
KRT contributed to the majority of work including preparation of Tn-PS A1 constructs, murine immunizations, production and isolation of Kt-IgM-8, ELISA, FACS, CDC assay, and SCID mice studies. KAK expressed and purified Kt-IgM-8 for SDS-PAGE and prepared samples for glycan arrays. MS synthesized the Tn antigen used for conjugation. J-PB assisted in the SCID mice studies. JMP conducted glycan array experiments. DTD conducted glycan array experiments. PRA is the principle investigator and director of the research.
Corresponding author
Ethics declarations
Conflict of interest
Kevin R. Trabbic, Kristopher A. Kleski, Mengchao Shi, Jean-Paul Bourgault, and Peter R. Andreana declare that they have no conflict of interest. The glycan array described is owned by and/or licensed to Siamab Therapeutics, Inc. in which Jillian M. Prendergast and Daniel T. Dransfield have a business and/or financial interest.
Ethical approval and ethical standards
C57BL/6 male mice (6 weeks) were obtained from Jackson Laboratories and maintained by the DLAR at the University of Toledo. All animal protocols were approved and performed in compliance with the relevant laws and institutional guidelines set forth by the IACUC of the University of Toledo (Protocol 107956). SHO® 4-week-old female mice (Crl:SHO®-PrkdcscidHrhr) were obtained from Charles River Laboratories and maintained by the DLAR at the University of Toledo. All animal protocols were approved and performed in compliance with the relevant laws and institutional guidelines set forth by the IACUC of the University of Toledo (Protocol Number 108420). The Sp2/0-Ag14 (ATCC CRL 1581) cell line was purchased from the American-Type Culture Collection. The MCF-7 (ATCC HTB-22) cell line and the HCT-116 (ATCC CCL-247) cell line was from Henry Ford Health Systems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Trabbic, K.R., Kleski, K.A., Shi, M. et al. Production of a mouse monoclonal IgM antibody that targets the carbohydrate Thomsen-nouveau cancer antigen resulting in in vivo and in vitro tumor killing. Cancer Immunol Immunother 67, 1437–1447 (2018). https://doi.org/10.1007/s00262-018-2206-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2206-0