Abstract
Background
Solid renal masses are often indeterminate for benignity versus malignancy on magnetic resonance imaging. Such masses are typically evaluated with either percutaneous biopsy or surgical resection. Percutaneous biopsy can be non-diagnostic and some surgically resected lesions are inadvertently benign.
Purpose
To assess the performance of ten machine learning (ML) algorithms trained with MRI-based radiomics features in distinguishing benign from malignant solid renal masses.
Methods
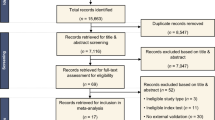
Patients with solid renal masses identified on pre-intervention MRI were curated from our institutional database. Masses with a definitive diagnosis via imaging (for angiomyolipomas) or via biopsy or surgical resection (for oncocytomas or renal cell carcinomas) were selected. Each mass was segmented for both T2- and post-contrast T1-weighted images. Radiomics features were derived from the segmented masses for each imaging sequence. Ten ML algorithms were trained with the radiomics features gleaned from each MR sequence, as well as the combination of MR sequences.
Results
In total, 182 renal masses in 160 patients were included in the study. The support vector machine algorithm trained on radiomics features from T2-weighted images performed superiorly, with an accuracy of 0.80 and an area under the curve (AUC) of 0.79. Linear discriminant analysis (accuracy = 0.84 and AUC = 0.77) and logistic regression (accuracy = 0.78 and AUC = 0.78) algorithms trained on T2-based radiomics features performed similarly. ML algorithms trained on radiomics features from post-contrast T1-weighted images or the combination of radiomics features from T2- and post-contrast T1-weighted images yielded lower performance.
Conclusion
Machine learning models trained with radiomics features derived from T2-weighted images can provide high accuracy for distinguishing benign from malignant solid renal masses.
Clinical impact
Machine learning models derived from MRI-based radiomics features may improve the clinical management of solid renal masses and have the potential to reduce the frequency with which benign solid renal masses are biopsied or surgically resected.



Similar content being viewed by others
References
V. E. Reuter and J. C. Presti, “Contemporary approach to the classification of renal epithelial tumors.,” Seminars in oncology, vol. 27, no. 2, pp. 124–37, Apr. 2000.
S. Collins, J. McKiernan, and J. Landman, “Update on the epidemiology and biology of renal cortical neoplasms.,” Journal of endourology, vol. 20, no. 12, pp. 975–85, Dec. 2006, https://doi.org/10.1089/end.2006.20.975.
S. K. Kang, W. C. Huang, P. V. Pandharipande, and H. Chandarana, “Solid renal masses: What the numbers tell us,” American Journal of Roentgenology, vol. 202, no. 6, pp. 1196–1206, 2014, https://doi.org/10.2214/AJR.14.12502.
J. J. Tomaszewski, R. G. Uzzo, and M. C. Smaldone, “Heterogeneity and renal mass biopsy: a review of its role and reliability.,” Cancer biology & medicine, vol. 11, no. 3, pp. 162–72, Sep. 2014, https://doi.org/10.7497/j.issn.2095-3941.2014.03.002.
S. C. Campbell et al., “Guideline for Management of the Clinical T1 Renal Mass,” Journal of Urology, vol. 182, no. 4 SUPPL., pp. 1271–1279, 2009, https://doi.org/10.1016/j.juro.2009.07.004.
D. C. Johnson et al., “Preoperatively misclassified, surgically removed benign renal masses: a systematic review of surgical series and United States population level burden estimate.,” The Journal of urology, vol. 193, no. 1, pp. 30–5, Jan. 2015, https://doi.org/10.1016/j.juro.2014.07.102.
F. U. Kay et al., “Diagnostic Performance and Interreader Agreement of a Standardized MR Imaging Approach in the Prediction of Small Renal Mass Histology.,” Radiology, vol. 287, no. 2, pp. 543–553, 2018, https://doi.org/10.1148/radiol.2018171557.
A. D. de Leon, P. Kapur, and I. Pedrosa, “Radiomics in Kidney Cancer: MR Imaging,” Magnetic Resonance Imaging Clinics of North America, vol. 27, no. 1, pp. 1–13, 2019, doi: https://doi.org/10.1016/j.mric.2018.08.005.
J. Mühlbauer et al., “Radiomics in Renal Cell Carcinoma-A Systematic Review and Meta-Analysis.,” Cancers, vol. 13, no. 6, pp. 1–15, Mar. 2021, https://doi.org/10.3390/cancers13061348.
A. Bhandari, M. Ibrahim, C. Sharma, R. Liong, S. Gustafson, and M. Prior, “CT-based radiomics for differentiating renal tumours: a systematic review.,” Abdominal radiology (New York), vol. 46, no. 5, pp. 2052–2063, 2021, https://doi.org/10.1007/s00261-020-02832-9.
J. Uhlig et al., “Radiomic Features and Machine Learning for the Discrimination of Renal Tumor Histological Subtypes: A Pragmatic Study Using Clinical-Routine Computed Tomography.,” Cancers, vol. 12, no. 10, pp. 1–14, Oct. 2020, https://doi.org/10.3390/cancers12103010.
D. Said et al., “Characterization of solid renal neoplasms using MRI-based quantitative radiomics features,” Abdominal Radiology, vol. 45, no. 9, pp. 2840–2850, 2020, https://doi.org/10.1007/s00261-020-02540-4.
E. R. DeLong, D. M. DeLong, and D. L. Clarke-Pearson, “Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach,” Biometrics, vol. 44, no. 3, 1988, https://doi.org/10.2307/2531595.
R. H. Thompson et al., “Tumor size is associated with malignant potential in renal cell carcinoma cases.,” The Journal of urology, vol. 181, no. 5, pp. 2033–6, May 2009, https://doi.org/10.1016/j.juro.2009.01.027.
G. M. Israel, N. Hindman, E. Hecht, and G. Krinsky, “The use of opposed-phase chemical shift MRI in the diagnosis of renal angiomyolipomas.,” AJR. American journal of roentgenology, vol. 184, no. 6, pp. 1868–72, Jun. 2005, https://doi.org/10.2214/ajr.184.6.01841868.
E. A. Lassel, R. Rao, C. Schwenke, S. O. Schoenberg, and H. J. Michaely, “Diffusion-weighted imaging of focal renal lesions: a meta-analysis.,” European radiology, vol. 24, no. 1, pp. 241–9, Jan. 2014, https://doi.org/10.1007/s00330-013-3004-x.
H. S. Yu et al., “Texture analysis as a radiomic marker for differentiating renal tumors,” Abdominal Radiology, vol. 42, no. 10, pp. 2470–2478, 2017, https://doi.org/10.1007/s00261-017-1144-1.
M. G. Lubner, “Radiomics and Artificial Intelligence for Renal Mass Characterization,” Radiologic Clinics of North America, vol. 58, no. 5, pp. 995–1008, 2020, https://doi.org/10.1016/j.rcl.2020.06.001.
U. N. Hoang et al., “Assessment of multiphasic contrast-enhanced mr textures in differentiating small renal mass subtypes,” Abdominal Radiology, vol. 43, no. 12, pp. 3400–3409, 2018, https://doi.org/10.1007/s00261-018-1625-x.
W. Wang, K. M. Cao, S. M. Jin, X. L. Zhu, J. H. Ding, and W. J. Peng, “Differentiation of renal cell carcinoma subtypes through MRI-based radiomics analysis,” European Radiology, vol. 30, no. 10, pp. 5738–5747, 2020, https://doi.org/10.1007/s00330-020-06896-5.
A. Goyal et al., “Role of MR texture analysis in histological subtyping and grading of renal cell carcinoma: a preliminary study,” Abdominal Radiology, vol. 44, no. 10, pp. 3336–3349, 2019, https://doi.org/10.1007/s00261-019-02122-z.
A. Razik et al., “MR texture analysis in differentiating renal cell carcinoma from lipid-poor angiomyolipoma and oncocytoma.,” The British journal of radiology, vol. 93, no. 1114, p. 20200569, Oct. 2020, https://doi.org/10.1259/bjr.20200569.
Q. Xu et al., “Differentiating Benign from Malignant Renal Tumors Using T2- and Diffusion-Weighted Images: A Comparison of Deep Learning and Radiomics Models Versus Assessment from Radiologists,” Journal of magnetic resonance imaging : JMRI, p. jmri.27900, Aug. 2021, https://doi.org/10.1002/jmri.27900.
I. L. Xi et al., “Deep learning to distinguish benign from malignant renal lesions based on routine MR imaging,” Clinical Cancer Research, vol. 26, no. 8, pp. 1944–1952, 2020, https://doi.org/10.1158/1078-0432.CCR-19-0374.
J. E. van Timmeren, D. Cester, S. Tanadini-Lang, H. Alkadhi, and B. Baessler, “Radiomics in medical imaging-"how-to" guide and critical reflection.,” Insights into imaging, vol. 11, no. 1, p. 91, Aug. 2020, https://doi.org/10.1186/s13244-020-00887-2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Massa’a, R.N., Stoeckl, E.M., Lubner, M.G. et al. Differentiation of benign from malignant solid renal lesions with MRI-based radiomics and machine learning. Abdom Radiol 47, 2896–2904 (2022). https://doi.org/10.1007/s00261-022-03577-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03577-3