Abstract
Purpose
We aimed to determine a new strategy for Liver Imaging Reporting and Data System category M (LR-M) criteria to improve the diagnosis of HCC ≤ 3.0 cm on magnetic resonance imaging (MRI).
Methods
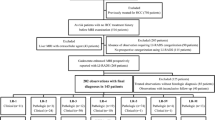
A total of 463 pathologically confirmed hepatic observations ≤ 3.0 cm (375 HCCs, 32 other malignancies, 56 benignities) in 384 patients at risk of HCC who underwent gadoxetate-enhanced MRI were retrospectively analyzed. Two radiologists evaluated the presence of major, ancillary, and LR-M features according to LI-RADS v2018. Of the ten LR-M features, those significantly associated with non-HCC malignancy were identified using multivariable logistic regression analysis, and new LR-M criteria for improving the diagnosis of HCC were investigated. Generalized estimating equations were used to compare sensitivity and specificity of LR-5 for diagnosing HCC using the new LR-M criteria with values calculated using the original LR-M criteria. p < 0.05 was considered to indicate a significant difference.
Results
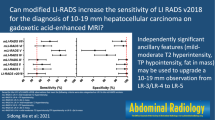
Of ten LR-M features, rim arterial-phase hyperenhancement, delayed central enhancement, targetoid restriction, and targetoid transitional-phase/hepatobiliary-phase appearance were independently significantly associated with non-HCC malignancy (adjusted odds ratio ≥ 6.2; p ≤ 0.02). Using the new LR-M criteria (two or more of these significant features), the sensitivity of LR-5 for diagnosing HCC was higher than that with the original LR-M criteria (69% [95% confidence interval 64–73%] vs. 65% [61–70%], p = 0.002), whereas the specificity was similar (90% [82–95%] vs. 92% [83–96%], p = 0.28).
Conclusion
The new LR-M criteria (two or more significant features) can improve the sensitivity of LR-5 for diagnosing HCC ≤ 3.0 cm, without compromising specificity.
Graphical abstract



Similar content being viewed by others
Abbreviations
- APHE:
-
Arterial-phase hyperenhancement
- CCA:
-
Intrahepatic cholangiocarcinoma
- cHCC-CCA:
-
Combined hepatocellular-cholangiocarcinoma
- CT:
-
Computed tomography
- HBP:
-
Hepatobiliary-phase
- HCC:
-
Hepatocellular carcinoma
- LI-RADS:
-
Liver Imaging Reporting and Data System
- MRI:
-
Magnetic resonance imaging
- NPV:
-
Negative predictive value
- OR:
-
Odds ratio
- PPV:
-
Positive predictive value
- TP:
-
Transitional phase
References
Mitchell DG, Bruix J, Sherman M, Sirlin CB. (2015) LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 61:1056-1065. https://doi.org/10.1002/hep.27304
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. (2018) Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723-750. https://doi.org/10.1002/hep.29913
American College of Radiology. (2018) CT/MRI LI-RADS v2018. Available via https://www.acrorg/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Corepdf
Huang B, Wu L, Lu XY, Xu F, Liu CF, Shen WF, et al. (2016) Small Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma in Cirrhotic Livers May Share Similar Enhancement Patterns at Multiphase Dynamic MR Imaging. Radiology 281:150-157. https://doi.org/10.1148/radiol.2016151205
Fowler KJ, Sheybani A, Parker RA, 3rd, Doherty S, E MB, Chapman WC, et al. (2013) Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. AJR Am J Roentgenol 201:332-339. https://doi.org/10.2214/ajr.12.9488
Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. (2014) Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 60:1268-1289. https://doi.org/10.1016/j.jhep.2014.01.021
Fowler KJ, Potretzke TA, Hope TA, Costa EA, Wilson SR. (2018) LI-RADS M (LR-M): definite or probable malignancy, not specific for hepatocellular carcinoma. Abdom Radiol (NY) 43:149-157. https://doi.org/10.1007/s00261-017-1196-2
Choi SH, Byun JH, Lim YS, Yu E, Lee SJ, Kim SY, et al. (2016) Diagnostic criteria for hepatocellular carcinoma ⩽3 cm with hepatocyte-specific contrast-enhanced magnetic resonance imaging. J Hepatol 64:1099-1107. https://doi.org/10.1016/j.jhep.2016.01.018
Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS, Kim SH, et al. (2012) Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology 264:761-770. https://doi.org/10.1148/radiol.12112517
Kim DH, Choi SH, Park SH, Kim KW, Byun JH, Kim SY, et al. (2019) Meta-analysis of the accuracy of Liver Imaging Reporting and Data System category 4 or 5 for diagnosing hepatocellular carcinoma. Gut 68:1719-1721. https://doi.org/10.1136/gutjnl-2019-318555
van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh JP, Bashir MR, et al. (2019) Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy-A Systematic Review. Gastroenterology 156:976-986. https://doi.org/10.1053/j.gastro.2018.11.020
Kim DH, Choi SH, Park SH, Kim KW, Byun JH, Kim SY, et al. (2020) Liver imaging reporting and data system category M: A systematic review and meta-analysis. Liver Int 40:1477-1487. https://doi.org/10.1111/liv.14420
Kim MY, Joo I, Kang HJ, Bae JS, Jeon SK, Lee JM. (2020) LI-RADS M (LR-M) criteria and reporting algorithm of v2018: diagnostic values in the assessment of primary liver cancers on gadoxetic acid-enhanced MRI. Abdom Radiol (NY) 45:2440-2448. https://doi.org/10.1007/s00261-020-02545-z
Park HJ, Kim YK, Cha DI, Ko SE, Kim S, Lee ES, et al. (2020) Targetoid hepatic observations on gadoxetic acid-enhanced MRI using LI-RADS version 2018: emphasis on hepatocellular carcinomas assigned to the LR-M category. Clin Radiol 75:478.e413-478.e423. https://doi.org/10.1016/j.crad.2020.01.002
Shao S, Liang Y, Kuang S, Chen J, Shan Q, Yang H, et al. (2020) Diagnostic performance of LI-RADS version 2018 in differentiating hepatocellular carcinoma from other hepatic malignancies in patients with hepatitis B virus infection. Bosn J Basic Med Sci 20:401–410. https://doi.org/10.17305/bjbms.2019.4576
Kim SS, Lee S, Choi JY, Lim JS, Park MS, Kim MJ. (2020) Diagnostic performance of the LR-M criteria and spectrum of LI-RADS imaging features among primary hepatic carcinomas. Abdom Radiol (NY) 45:3743-3754. https://doi.org/10.1007/s00261-020-02562-y
Llovet JM, Fuster J, Bruix J. (2004) The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl 10:S115-120. https://doi.org/10.1002/lt.20034
Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, et al. (2005) Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology 234:954-960. https://doi.org/10.1148/radiol.2343040153
Bland JM. (2004) Cluster randomised trials in the medical literature: two bibliometric surveys. BMC Med Res Methodol 4:21. https://doi.org/10.1186/1471-2288-4-21
Zhang Z. (2018) Estimating the optimal cutoff point for logistic regression. Open Access Theses & Dissertations. https://digitalcommons.utep.edu/open_etd/1565
Kim YY, Kim MJ, Kim EH, Roh YH, An C. (2019) Hepatocellular Carcinoma versus Other Hepatic Malignancy in Cirrhosis: Performance of LI-RADS Version 2018. Radiology 291:72-80. https://doi.org/10.1148/radiol.2019181995
Kobayashi M, Ikeda K, Saitoh S, Suzuki F, Tsubota A, Suzuki Y, et al. (2000) Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer 88:2471-2477. https://doi.org/10.1002/1097-0142(20000601)88:11<2471::aid-cncr7>3.0.co;2-t
Ferlan-Marolt V, Markovic S. (1986) Clinicomorphological manifestations of primary liver carcinoma (PLC) in liver cirrhosis. Cancer Detect Prev 9:491-493.
Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. (2008) Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 47:82-89. https://doi.org/10.1002/hep.21933
Acknowledgements
This study was financially supported by Bayer Korea, but the authors had complete control of the data and information submitted for publication at all times.
Funding
This study was financially supported by Bayer Korea, but the authors had complete control of the data and information submitted for publication at all times.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Sang Hyun Choi is an advisory board member of Bayer Korea and receives research funding from Bayer Korea, but the authors had complete control of the data and information submitted for publication at all times. The other authors have nothing to declare.
Ethical approval
This retrospective study was approved by institutional review board of Asan Medical Center (IRB No. 2019-1556).
Informed consent
The IRB waived informed consent for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jang, J.K., Choi, S.H., Byun, J.H. et al. New strategy for Liver Imaging Reporting and Data System category M to improve diagnostic performance of MRI for hepatocellular carcinoma ≤ 3.0 cm. Abdom Radiol 47, 2289–2298 (2022). https://doi.org/10.1007/s00261-022-03538-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03538-w