Abstract
Background
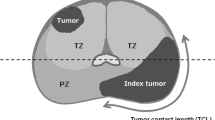
Extraprostatic extension (EPE) of prostate cancer is associated with a poor prognosis. The broad-based capsule-tumor interface has been recognized as one of the worrisome imaging features in multiparametric prostate MRI (mpMRI). However, there was significant heterogeneity among the measurement method used in prior studies.
Objectives
This study’s objectives were to investigate and compare the accuracy between the curvilinear and linear measurement, find the optimal cut-off contact surface threshold for the diagnosis of EPE, and assess the benefit of the additional contact surface measurement versus visual assessment alone.
Methods
The status of EPE in mpMRI and the overall PI-RADS were assessed. The tumor’s dimensions, the actual tumor-capsule contact length (ACTCL), and the absolute tumor-capsule contact length (ABTCL) were measured. The parameters were analyzed and correlated with the EPE status from prostatectomy specimens.
Results
Ninety-five patients who underwent mpMRI followed by prostatectomy were included in the study. High Gleason score (score 8–9), radiologist’s impression of EPE, and PI-RADS 5 were significantly correlated with EPE in surgical specimens (p = 0.014, p < 0.001, and p < 0.001, respectively). Both ACTCL and ABTCL of patients with EPE were significantly higher than those without EPE in all imaging sequences (p < 0.001 to p = 0.003). The ABTCL has higher accuracy than the ACTCL. Dynamic contrast enhancement (DCE) was the most accurate sequence to measure the contact interface. The recommended cut-off value of ABTCL was 15.0 mm, which had a sensitivity and specificity of 75.86% and 72.09%. Multivariable analysis revealed that the ABTCL > 15 mm and the radiologist’s impression on visual assessment were the only two independent predictors for the prediction of EPE (p = 0.048 and p = 0.016, respectively). Improvement of diagnostic performance was achieved when the two factors were combined.
Conclusion
The ABTCL has better accuracy than the curvilinear measurement in the prediction of EPE. The optimum sequence for the measurement of the contact surface is the DCE. We recommended using 15.0 mm as a cut-off point.
Clinical impact
The addition of the ABTCL measurement showed an increase in diagnostic performance. We encourage radiologists to use the capsular contact measurement in addition to their visual assessment to detect EPE in pre-operative MRI.



Similar content being viewed by others
References
Rawla P (2019) Epidemiology of Prostate Cancer. World J Oncol 10:63–89. https://doi.org/https://doi.org/10.14740/wjon1191
Buyyounouski MK, Choyke PL, McKenney JK, et al (2017) Prostate Cancer – Major Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin 67:245–253. https://doi.org/https://doi.org/10.3322/caac.21391
Li J, Djenaba JA, Soman A, et al (2012) Recent Trends in Prostate Cancer Incidence by Age, Cancer Stage, and Grade, the United States, 2001–2007. Prostate Cancer 2012:1–8. https://doi.org/https://doi.org/10.1155/2012/691380
Cotter K, Konety B, Ordonez MA (2016) Contemporary Management of Prostate Cancer. F1000Res 5:179. https://doi.org/https://doi.org/10.12688/f1000research.7183.1
Akduman B, Crawford ED (2006) Treatment of localized prostate cancer. Rev Urol 8 Suppl 2:S15–S21
Weiner AB, Nettey OS, Morgans AK (2019) Management of Metastatic Hormone-Sensitive Prostate Cancer (mHSPC): an Evolving Treatment Paradigm. Curr Treat Options in Oncol 20:69. https://doi.org/https://doi.org/10.1007/s11864-019-0668-8
Keyes M, Crook J, Morton G, et al (2013) Treatment options for localized prostate cancer. Can Fam Physician 59:1269–1274
Ritch C, Cookson M (2018) Recent trends in the management of advanced prostate cancer. F1000Res 7:. https://doi.org/https://doi.org/10.12688/f1000research.15382.1
Cornford P, van den Bergh RCN, Briers E, et al (2020) EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II—2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. European Urology. https://doi.org/https://doi.org/10.1016/j.eururo.2020.09.046
Mottet N, Bellmunt J, Bolla M, et al (2017) EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. European Urology 71:618–629. https://doi.org/https://doi.org/10.1016/j.eururo.2016.08.003
Park SY, Cho NH, Jung DC, Oh YT (2018) Prostate Imaging-Reporting and Data System Version 2: Beyond Prostate Cancer Detection. Korean J Radiol 19:193–200. https://doi.org/https://doi.org/10.3348/kjr.2018.19.2.193
Boesen L (2017) Multiparametric MRI in detection and staging of prostate cancer. Dan Med J 64:B5327.
Bjurlin MA, Rosenkrantz AB, Taneja SS (2017) Role of MRI prebiopsy in men at risk for prostate cancer: taking off the blindfold. Curr Opin Urol 27:246–253. https://doi.org/https://doi.org/10.1097/MOU.0000000000000389
Schoots IG, Roobol MJ, Nieboer D, et al (2015) Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 68:438–450. https://doi.org/https://doi.org/10.1016/j.eururo.2014.11.037
Hole KH, Axcrona K, Lie AK, et al (2013) Routine pelvic MRI using phased-array coil for detection of extraprostatic tumour extension: accuracy and clinical significance. Eur Radiol 23:1158–1166. https://doi.org/https://doi.org/10.1007/s00330-012-2669-x
Kapoor J, Namdarian B, Pedersen J, et al (2013) Extraprostatic extension into periprostatic fat is a more important determinant of prostate cancer recurrence than an invasive phenotype. J Urol 190:2061–2066. https://doi.org/https://doi.org/10.1016/j.juro.2013.06.050
Ball MW, Partin AW, Epstein JI (2015) Extent of extraprostatic extension independently influences biochemical recurrence-free survival: evidence for further pT3 subclassification. Urology 85:161–164. https://doi.org/https://doi.org/10.1016/j.urology.2014.08.025
Boesen L, Chabanova E, Løgager V, et al (2015) Prostate cancer staging with extracapsular extension risk scoring using multiparametric MRI: a correlation with histopathology. Eur Radiol 25:1776–1785. https://doi.org/https://doi.org/10.1007/s00330-014-3543-9
Turkbey B, Rosenkrantz AB, Haider MA, et al (2019) Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. European Urology 76:340–351. https://doi.org/https://doi.org/10.1016/j.eururo.2019.02.033
Zapała P, Dybowski B, Bres-Niewada E, et al (2019) Predicting side-specific prostate cancer extracapsular extension: a simple decision rule of PSA, biopsy, and MRI parameters. Int Urol Nephrol 51:1545–1552. https://doi.org/https://doi.org/10.1007/s11255-019-02195-1
Pesapane F, Standaert C, Visschere PD, Villeirs G (2020) T-staging of prostate cancer: Identification of useful signs to standardize detection of posterolateral extraprostatic extension on prostate MRI. Clinical Imaging 59:1–7. https://doi.org/https://doi.org/10.1016/j.clinimag.2019.08.007
Mehralivand S, Shih JH, Harmon S, et al (2019) A Grading System for the Assessment of Risk of Extraprostatic Extension of Prostate Cancer at Multiparametric MRI. Radiology 290:709–719. https://doi.org/https://doi.org/10.1148/radiol.2018181278
Krishna S, Lim CS, McInnes MDF, et al (2018) Evaluation of MRI for diagnosis of extraprostatic extension in prostate cancer. Journal of Magnetic Resonance Imaging 47:176–185. https://doi.org/https://doi.org/10.1002/jmri.25729
Bakir B, Onay A, Vural M, et al (2019) Can Extraprostatic Extension Be Predicted by Tumor-Capsule Contact Length in Prostate Cancer? Relationship With International Society of Urological Pathology Grade Groups. American Journal of Roentgenology 214:588–596. https://doi.org/https://doi.org/10.2214/AJR.19.21828
Kim T-H, Woo S, Han S, et al (2020) The Diagnostic Performance of the Length of Tumor Capsular Contact on MRI for Detecting Prostate Cancer Extraprostatic Extension: A Systematic Review and Meta-Analysis. Korean J Radiol 21:684–694. https://doi.org/https://doi.org/10.3348/kjr.2019.0842
Krishna S, Lim CS, McInnes MDF, et al (2018) Evaluation of MRI for diagnosis of extraprostatic extension in prostate cancer. J Magn Reson Imaging 47:176–185. https://doi.org/https://doi.org/10.1002/jmri.25729
Baco E, Rud E, Vlatkovic L, et al (2015) Predictive value of magnetic resonance imaging determined tumor contact length for extracapsular extension of prostate cancer. J Urol 193:466–472. https://doi.org/https://doi.org/10.1016/j.juro.2014.08.084
Tourinho-Barbosa R, Srougi V, Nunes-Silva I, et al (2018) Biochemical recurrence after radical prostatectomy: what does it mean? Int Braz J Urol 44:14–21. https://doi.org/https://doi.org/10.1590/S1677-5538.IBJU.2016.0656
Kir G, Arikan EA, Seneldir H, et al (2020) Determining the cut-off values of tumor diameter, degree of extraprostatic extension, and extent of surgical margin positivity with regard to biochemical recurrence of prostate cancer after radical prostatectomy. Ann Diagn Pathol 44:151431. https://doi.org/https://doi.org/10.1016/j.anndiagpath.2019.151431
Farchoukh L, Laframboise WA, Nelson JB, et al (2020) Multifocal Extraprostatic Extension of Prostate Cancer. Am J Clin Pathol 153:548–553. https://doi.org/https://doi.org/10.1093/ajcp/aqz193
Salerno J, Finelli A, Morash C, et al (2016) Multiparametric magnetic resonance imaging for pre-treatment local staging of prostate cancer: A Cancer Care Ontario clinical practice guideline. Can Urol Assoc J 10:E332–E339. https://doi.org/https://doi.org/10.5489/cuaj.3823
Park SY, Kim JJ, Kim TH, et al (2010) The Role of Endorectal Magnetic Resonance Imaging in Predicting Extraprostatic Extension and Seminal Vesicle Invasion in Clinically Localized Prostate Cancer. Korean J Urol 51:308–312. https://doi.org/https://doi.org/10.4111/kju.2010.51.5.308
Rud E, Klotz D, Rennesund K, et al (2015) Preoperative magnetic resonance imaging for detecting uni- and bilateral extraprostatic disease in patients with prostate cancer. World J Urol 33:1015–1021. https://doi.org/https://doi.org/10.1007/s00345-014-1362-x
Dominguez C, Plata M, Cataño JG, et al (2018) Diagnostic accuracy of multiparametric magnetic resonance imaging in detecting extracapsular extension in intermediate and high - risk prostate cancer. Int Braz J Urol 44:688–696. https://doi.org/https://doi.org/10.1590/S1677-5538.IBJU.2016.0485
Hansen NL, Koo BC, Gallagher FA, et al (2017) Comparison of initial and tertiary centre second opinion reads of multiparametric magnetic resonance imaging of the prostate prior to repeat biopsy. Eur Radiol 27:2259–2266. https://doi.org/https://doi.org/10.1007/s00330-016-4635-5
Sonn GA, Fan RE, Ghanouni P, et al (2019) Prostate Magnetic Resonance Imaging Interpretation Varies Substantially Across Radiologists. European Urology Focus 5:592–599. https://doi.org/https://doi.org/10.1016/j.euf.2017.11.010
Kongnyuy M, Sidana A, George AK, et al (2017) Tumor contact with prostate capsule on magnetic resonance imaging: A potential biomarker for staging and prognosis. Urol Oncol 35:30.e1-30.e8. https://doi.org/https://doi.org/10.1016/j.urolonc.2016.07.013
Rud E, Diep L, Baco E (2018) A prospective study evaluating indirect MRI-signs for the prediction of extraprostatic disease in patients with prostate cancer: tumor volume, tumor contact length and tumor apparent diffusion coefficient. World J Urol 36:629–637. https://doi.org/https://doi.org/10.1007/s00345-018-2171-4
Onay A, Vural M, Armutlu A, et al (2019) Evaluation of the most optimal multiparametric magnetic resonance imaging sequence for determining pathological length of capsular contact. Eur J Radiol 112:192–199. https://doi.org/https://doi.org/10.1016/j.ejrad.2019.01.020
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eurboonyanun, K., Pisuchpen, N., O’Shea, A. et al. The absolute tumor-capsule contact length in the diagnosis of extraprostatic extension of prostate cancer. Abdom Radiol 46, 4014–4024 (2021). https://doi.org/10.1007/s00261-021-03063-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03063-2