Abstract
Objectives
To determine the accuracy and assess the clinical significance of surface-coil 1.5-T magnetic resonance imaging (MRI) for the detection of locally advanced prostate cancer (PCa).
Methods
Between December 2007 and January 2010, we examined 209 PCa patients (mean age = 62.5 years) who were consecutively treated with robot-assisted laparoscopic prostatectomy and prospectively staged by MRI. One hundred and thirty-five patients (64.6 %) had locally advanced disease. Conventional clinical tumour stage and MRI-assessed tumour stage were compared with histopathological tumour stage (pT). Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and overall accuracy (OA) were calculated using pT as the “gold standard”. Overstaged and understaged cases at MRI were reviewed.
Results
Sensitivity, specificity, PPV, NPV and OA for the detection of locally advanced disease were 25.9, 95.9, 92.1, 41.2 and 50.5 % and 56.3, 82.2, 85.4, 50.4 and 65.4 % for clinical staging and MRI, respectively. Among patients understaged at MRI, the resection margins were free in 64.4 % of the cases (38/59).
Conclusions
Although the accuracy was limited, the detection of locally advanced disease improved substantially when MRI was added to routine clinical staging. The majority of the understaged patients nevertheless achieved free margins. When assessing the clinical significance of MRI staging the extent of extraprostatic extension has to be considered.
Key Points
• MRI substantially improves detection of locally advanced prostate cancer
• MRI has limited overall staging accuracy
• Most T3 cancers unrecognised at MRI still achieved free resection margins
• Assessing the true clinical contribution of MRI remains challenging
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reliable identification of locally advanced prostate cancer (PCa) is regarded crucial for making decisions on treatments [1]. Traditionally, clinical evaluation of the extent of PCa has been based on prostate-specific antigen (PSA), digital rectal examination (DRE), transrectal ultrasound (TRUS) and biopsy results [1]. These clinical tools often underestimate tumour extent and are not accurate enough to recognise locally advanced disease [1–4]. For other pelvic malignancies, in particular rectal cancer, magnetic resonance imaging (MRI) is the “gold standard” for detecting locally advanced disease and for treatment stratification [5]. MRI is increasingly being used and recommended for PCa staging [6–11]. However, a wide range of sensitivities and specificities for staging have been reported [8, 11]. The use of endorectal phased-array coil has been shown to significantly improve the specificity of extraprostatic extension (EPE) detection, but not the sensitivity when compared with pelvic phased-array coil with equal image resolution [12]. Furthermore, use of an endorectal coil is associated with increased costs, reduced patient throughput and discomfort to the patient. The performance of MRI is limited by the facts that low-grade tumours are hardly distinguishable from normal prostate tissue [11], that malignant glandular tissue often is intermixed with benign prostate tissue [13, 14], and that the typical spatial resolution does not allow for the detection of sub-millimetre disease [15].
There is currently an ongoing debate regarding the clinical usefulness of PCa staging by MRI [9, 11, 16, 17]. The main benefit of MRI staging probably lies in the discovery of clinically unrecognised, locally advanced cancer. Being a third-line referral cancer hospital with a significant proportion of patients with locally advanced disease, our study population is in this sense appropriate [18] for the assessment of the clinical usefulness of MRI staging.
In this study, we explored the performance of clinical routine pelvic MRI using phased-array coil and a widely applicable imaging protocol for the detection of locally advanced PCa. Consecutive and prospectively performed MRI and histopathology interpretations were compared without re-assessments or patient exclusions to ensure that the reported data truly reflected routine clinical practice. We also sought to assess clinical significance by reviewing overstaged and understaged cases and by recording the resection margin status.
Materials and methods
Patients
Between December 2007 and January 2010, 209 PCa patients with preoperative MRI performed in our hospital who had been consecutively stratified for surgical treatment were included. Patients considered for radical prostatectomy had no evidence of skeletal metastases, either at skeletal scintigraphy (PSA > 10 ng/ml) or at MRI. Patients preoperatively treated with radiation therapy or androgen deprivation were excluded. Clinical data, including T-stage (cT) deduced from routine DRE and TRUS performed at referral hospitals and histopathological T-stage (pT) are summarised in Table 1. The regional ethics committee approved the study and granted a waiver of informed consent for this single-institutional retrospective study of prospectively recorded data.
MRI
Median number of days between biopsy or TUR-P and MRI was 89 days (range = 14–1,621 days). All patients underwent MRI using a 1.5-T Siemens ESPREE (Siemens, Erlangen, Germany) magnet and phased-array coils. An endorectal coil was not used. The MRI sequences and imaging parameters are summarised in Table 2. T-stage was deduced from two different T2-weigthed (T2W) sequences: transversal 3D SPACE with isotropic voxels of 1 mm and transversal 2D TSE with a native in-plane resolution of 0.6 × 0.6 mm2 and slice thickness of 3–4 mm. For some patients, additional coronal T2W images were acquired. Diffusion-weighted (DW) MRI of the prostate and seminal vesicles, along with the T2W morphological images, were used for detecting tumours and assessing their extent. DW MRI with multiple b values (50-300-500-1,000 mm2/s, 0-50-300-500-2,000 mm2/s or 50-500-1,000-1,500 mm2/s) and voxels of 2 × 2 × 4 mm3 were applied for diffusion quantification (apparent diffusion coefficient, ADC). For most patients, the protocol also included a heavily DW (b = 2,000 mm2/s) sequence with larger voxels (3 × 3 × 6 mm3) for qualitatively assessing tumour localisation and extent. Coronal 3D SPACE T1-weighted MR images with isotropic voxels of 1.1 mm covering the whole pelvis and lower abdomen were acquired. Peristalsis was suppressed by intravenous administration of 1 mg butyl scopolamine (Buscopan®; Boehringer Ingelheim, Germany) and intramuscular administration of 1 mg glucagon (Novo Nordisk, Bagsværd, Denmark).
The MR images were read by one of two experienced radiologists independently (K.H.H. and K.K). At the start of the study the radiologists (K.H.H. and K.K.) had 3 years of prostate MRI experience with approximately 300 and 100 examinations per year, respectively. Tumour stage was classified according to the 5th edition TNM classification [19]. The biopsy results were available for the radiologists when interpreting the MR images. Tumour in the prostate was predicted by low signal intensity on T2W and T1W (no haemorrhage) images and low ADC, with a correspondingly high signal intensity on heavily DW images (b ≥ 1,000) [9]. The criteria used for interpreting stage T3a were, in the presence of tumour, capsular bulging, stranding into the periprostatic fatty tissue or asymmetry of the nevrovascular bundle [20, 21]. Thickening of the prostate capsule without stranding or bulging beyond the confines of the prostate was staged as T2. The criteria for stage T3b, invasion of the seminal vesicles, were low signal intensity on T2W and T1W (no haemorrhage) images with a correspondingly high signal intensity on heavily DW images. Stage T4 was noted if the tumour extended into the bladder neck. In case of uncertainty, it was agreed that tumours should be downstaged.
MRI-assessed T-stage (mrT) data are summarised in Table 1. For ten patients, no tumour could be identified at MRI (mrT0). Movement and image artefacts made it impossible to assess tumour stages in two patients (mrTx). No patients were excluded from the analyses, either mrT0 or mrTx.
Robot-assisted laparoscopic prostatectomy
A three-armed robotic DaVinci® system (Intuitive Surgical, Sunnyvale, CA, USA) was used to perform robot-assisted laparoscopic radical prostatectomy (RALP). The operative positioning was in a steep Trendelenburg position (45º), and the surgical approach was based mainly on the Vattikutti Institute technique [22]. The median number of days between MRI and RALP was 81 (range = 0–358). Where MRI showed an EPE, a locally extended excision was performed.
Histopathological assessment of tumour stage
The specimens were coated with three different inks and fixed in 10 % buffered formaldehyde for at least 2 days. Grossing was performed according to a standardised protocol where total prostate with seminal vesicles was embedded. [23, 24]. The apex and base of the prostate was cut by the cone method as sagittal sections. The remaining body of the prostate was sectioned serially at 3–4 mm intervals in the transverse plane and prepared as whole-mount sections. The understaged specimens were examined by two experienced uropathologists (A.K.L. and L.V.). The extent of EPE was classified as focal or established using the method described by Sung and co-workers [25] using a cut-off at 1 mm for the radial distance of EPE. Histopathological staging was performed according to the 5th edition TNM classification [19] and the modified Gleason score system was used for assessing tumour aggressiveness [26].
Histopathological T-stage data are summarised in Table 1. For one patient, intraprostatic incision was present, hence the presence or absence of EPE could not be reliably assessed, and this patient was excluded from the analyses (pTx). Thus, the number of patients eligible for an assessment of MR performance was 208.
Data analysis
The histopathological tumour stage was compared with cT and mrT by using descriptive statistics. The comparison between MRI and histopathology was performed on a per patient basis. Diagnostic accuracy for assessing locally advanced disease was obtained by calculating sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and overall accuracy (OA). In the overstaged cases, the MRI examinations and reports were reviewed. In the understaged cases, the radial extent of EPE was quantified in the prostatectomy specimens if the surgical margin was positive.
Results
T-stage by DRE and TRUS
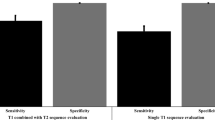
Staging accuracy is summarised in Fig. 1. A total of 35 out of 135 patients (25.9 %) with a histologically verified EPE were clinically recognised as T3. Specificity, PPV, NPV and OA for identifying locally advanced disease were 95.9, 92.1, 41.2 and 50.5 %, respectively. Locally advanced disease was found in 49 out of 96 patients (51.0 %) with clinical T1c. For patients with cT2 tumour, 51 of 74 patients (68.9 %) had more advanced disease than clinically anticipated.
T-stage by MRI
Staging accuracy is summarised in Fig. 2. MRI detected EPE in 76 of 135 patients (56.3 %) with locally advanced disease. Specificity, PPV, NPV and OA for the detection of locally advanced disease were 82.2, 85.4, 50.4 and 65.4 %, respectively.
MRI overstaging
In 13 cases (9.6 %) MRI indicated locally advanced disease that was not confirmed by histopathology (Fig. 2). In all these cases uncertainty regarding the presence or absence of EPE was noted in the MRI report. All patients had small glands and none showed post-biopsy haemorrhage.
MRI understaging
Fifty-nine patients with locally advanced disease were interpreted as having organ-confined disease at MRI (Fig. 2). Thirty-eight of these patients had a free resection margin (Fig. 3). The re-evaluation of the remaining 21 cases revealed four patients with focal EPE (< 1 mm) (Fig. 4), whereas 17 patients had established EPE exceeding 1 mm, (mean = 1.5 mm, range = 1–6 mm) (Fig. 5).
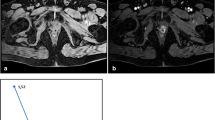
Focal extraprostatic extension. a T2W MRI with a heavily DW image of the prostate overlaid (in colour). b T2W close-up of the prostate. c Corresponding whole-mount section with a 1 mm2 grid overlay. d Close-up microscopic views; 40× magnification shows tumour islet (black arrow) representing focal extraprostatic extension of less than 1 mm radial in length, which was unrecognised and not clearly demonstrated (white arrow, b) at MRI
Established extraprostatic extension. a T2W MRI with a heavily DW image of the prostate overlaid (in colour). b T2W close-up of the prostate. c Corresponding whole-mount section with a 1 mm2 grid overlay. d Close-up microscopic view. The dashed line represents the boundary of the prostate and the black arrow indicates the extraprostatic extension of 2–3 mm in radial length, which was clearly demonstrated (white arrow, b) and correctly staged at MRI
Cumulative T3 detection performance
Cumulative performance for the detection of locally advanced PCa is summarised in Fig. 6. The detection rate from routine DRE and TRUS was 25.9 %. When preoperative MRI was added, the detection rate increased by 30.4 % (to 56.3 %). Out of all the patients who were understaged at MRI, 38 achieved a free resection margin (28.1 %), whereas 21 (15.6 %) did not.
Cumulative performance for detection of non-organ confined prostate cancer. The bar represents all 135 patients with extraprostatic extension (EPE). Separate percentages are given for digital rectal exploration (DRE) and transrectal ultrasound (TRUS) combined, for magnetic resonance imaging (MRI), and for those with unrecognised EPE with negative and positive resection margins
Discussion
This report shows that although limited in overall accuracy, the detection of locally advanced disease improved substantially when phased array 1.5-T MRI was added to clinical staging by routine DRE and TRUS. Furthermore, for most patients the misrecognition of EPE did not lead to a compromised surgical outcome.
The accuracy of the detection of locally advanced PCa in our study (65.4 %) was within the range, but at the lower end of previously published studies (59.4–85.9 %) [12, 27–31]. The wide range of reported accuracy levels reflects differences in patient populations, histopathological interpretations, evaluation methods, MR technology and reader experience; thus, comparisons of reported results and assessments of clinical significance are challenging. Even with a MRI protocol representing minimal technical requirements [9, 16] and a limited overall accuracy, the clinical significance of MRI staging in our study was evident, as EPE leading to a positive margin was overlooked in only 21 cases (15.6 %).
Patient populations influence staging accuracy, as a high rate of organ-confined disease may obscure a poor detection of locally advanced disease, and small study populations may result in higher staging performance [18]. Our study population was relatively large (n = 208) and consisted of 64.9 % with stage pT3-disease. Furthermore, we did not exclude examinations with unsatisfactory image qualities; hence, our results reflected routine radiological practice. A limitation to our study is the lack of sector-based comparison between MRI and histopathology. Neither the side nor the level of EPE was consistently reported in the prospectively recorded data.
In 2009 the International Society of Urological Pathology (ISUP) reached consensus that EPE should be stratified into focal (Fig. 4) or minimal, or established (Fig. 5) or extensive [32]. However, no consensus has been reached regarding the criteria of focal versus established EPE [1, 32, 33]. ISUP also reported considerable inconsistencies in the interpretation of EPE status in equivocal specimens. This inconsistency inevitably will influence the level of MRI accuracy as histopathological evaluation is considered the “gold standard”. In our study, a striking 64.4 % of the unrecognised T3 cases at MRI had free margins. When considering that extended surgery was not performed due to preoperative interpretation of organ-confined disease, free margins indicate that the extension of EPE was minimal (focal EPE). Consequently, by using established EPE [32, 33] as the histopathological criterion for clinically significant, locally advanced disease, MRI accuracy may improve.
Image resolution is critical for the visualisation of EPE. According to the Nyquist theorem, anatomical structures must be twice the sampling size (pixel) in order to be detected. The resolution used in our MR sequences does not allow for the reliable depiction of EPE with an extent less than approximately 1 mm. Millimetre resolution is also recommended for MR staging in other pelvic malignancies [5, 34, 35]. A radial extent of EPE that exceeds 1 mm has recently been reported to predict recurrence [36, 37]. Improved resolution using endorectal coil [12] and higher field strengths have been shown to enable the detection of EPE of 0.5 mm [12, 15]. However, very small voxels does not necessarily translate into improved detection of focal EPE, as minor involuntary movement of the prostate will induce partial volume effects. Furthermore, depicting EPE of less than 1 mm will not necessarily improve surgical outcome. Due to limited spatial resolution, dynamic contrast-enhanced MRI would probably not improve the detection of focal EPE [28].
In the case of equivocal EPE status at MRI, we agreed to downstage, and thus the overstaging rate was low (9.6 %). However, this practice led to understaging. Tumour admixed with extraprostatic fat or bladder musculature is characteristic of EPE, and microscopic tumour foci and focal EPE (< 1 mm) will not be detectable at MRI (Fig. 4). As a result, understaging is unavoidable. Therefore, if MRI is to be a useful decision-making tool for urologists, overstaging must be minimised. By using clearly visualised EPE as an MRI criterion for T3 disease, a high PPV (85.4 %) was achieved. The risk associated with systematic downstaging is that surgeons may wrongly assume that the nerve-sparing approach is safe.
When attempting to contextualise our MR findings, we realised that concerns related to pathology and surgery had to be considered. As the majority of patients with unrecognised locally advanced disease achieved free resection margin, the clinical significance of MRI is obviously not reflected solely by T-stage accuracy. In the light of recent reports that indicate the prognostic significance of radial EPE quantification [36, 37] and the limited ability of MRI to detect focal EPE, stratification of EPE as focal or established is of importance when assessing the clinical significance of MRI. If the clinical significance of MRI is assessed in terms of resection margin status, consideration must be given to both the extent of EPE and the surgical plane of dissection (preserving function versus ensuring free margin). Based on the results and limitations of our study, we are currently investigating in a prospective study (ClinicalTrials NCT01464216) the relationship between multiparametric MRI with increased spatial resolution, the radial extent of EPE at pathology, surgical techniques, resection margin status and long-term follow-ups.
In conclusion, although the detection accuracy was limited, MRI substantially improved the detection of locally advanced disease when added to conventional staging by routine DRE and TRUS. Strict MRI criteria for EPE ensured minimal overstaging, but induced understaging. Free resection margins were achieved for the majority of patients were MRI failed to recognise EPE. This indicates that the clinical significance of T-staging by MRI is not fully reflected by the ability to detect EPE per se. By stratifying the extent of EPE into focal or established, the staging accuracy is likely to increase as will the clinical significance of MRI as a tool for treatment planning.
Abbreviations
- DRE:
-
Digital rectal examination
- EPE:
-
Extraprostatic extension
- mrT:
-
MRI-assessed tumour stage
- DW:
-
Diffusion-weighted
- NPV:
-
Negative predictive value
- OA:
-
Overall accuracy
- PCa:
-
Prostate cancer
- PPV:
-
Positive predictive value
- PSA:
-
Prostate-specific antigen
- RALP:
-
Robot-assisted laparoscopic radical prostatectomy
- TRUS:
-
Transrectal ultrasound
References
European Association of Urology (2012) Guidelines on Prostate Cancer. EAU, Arnhem, the Netherlands. Avaliable via http://www.uroweb.org/guidelines/online-guidelines. Accessed 10 April 2012
Okihara K, Kamoi K, Lane RB, Evans RB, Troncoso P, Babaan RJ (2002) Role of systematic ultrasound-guided biopsies in predicting extraprostatic extension and seminal vesicle invasion in men with prostate cancer. J Clin Ultrasound 30:123–131
Öbek C, Louis P, Civantos F, Soloway MS (1999) Comparison of digital rectal examination and biopsy results with the radical prostatectomy specimen. J Urol 161:494–498
Smith JA, Scardino PT, Resnick MI, Hernandez AD, Rose SC, Egger MJ (1997) Transrectal ultrsound versus digital rectal examination for the staging of carcinoma of the prostate: results of a prospective, multi-international trial. J Urol 157:902–906
MERCURY Study Group (2006) Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 333(7572):779
Umbehr M, Bachmann LM, Held U et al (2009) Combined magnetic resonance imaging and magnetic resonance spectroscopy imaging in the diagnosis of prostate cancer: a systematic review and meta-analysis. Eur Urol 55:575–591
Verma S, Rajesh A (2011) A clinically relevant approach to imaging prostate cancer: review. AJR Am J Roentgenol 196:S1–S10
Turkbey B, Pinto PA, Choyke PL (2009) Imaging techniques for prostate cancer: implications for focal therapy. Nat Rev Urol 6:191–20
Barentsz JO, Richenberg J, Clements R et al (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22:746–757
Hoeks CMA, Barentsz JO, Hambrock T et al (2011) Prostate cancer: multiparametris MR imaging for detection, localization, and staging. Radiology 261:46–66
Kurhanewicz J, Vigneron D, Carroll P, Coakley F (2008) Multiparametric magnetic resonance imaging in cancer: present and future. Curr Opin Urol 18:71–77
Fütterer JJ, Engelbrecht MR, Jager GJ et al (2007) Prostate cancer: comparison of local staging accuracy of pelvic phased-array coil alone versus integrated endorectal-pelvic phased-array coils. Local staging accuracy of prostate cancer using endorectal coil MR imaging. Eur Radiol 17:1055–1065
Langer DL, van der Kwast TH, Evans AJ et al (2008) Intermixed normal tissue within prostate cancer: effect on MR imaging measurement of apparent diffusion coefficient and T2—sparse versus dense cancers. Radiology 249:900–908
Langer DL, van der Kwast TH, Evans AJ et al (2010) Prostate tissue composition and MR measurements: investigating the relationships between ADC, T2, K(trans), V(e), and corresponding histologic features. Radiology 255:485–494
Heijmink SW, Fütterer JJ, Hambrock T et al (2007) Prostate cancer: body-array versus endorectal coil MR imaging at 3T—comparison of image quality, localization, and staging performance. Radiology 244:184–195
Dickinson L, Ahmed HU, Allen C et al (2011) Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 59:477–494
Heidenreich A (2011) Consensus criteria for the use of magnetic resonance imaging in the diagnosis and staging of prostate cancer: not ready for routine use. Eur Urol 59:495–497
Brajtbord JS, Lavery HJ, Nabizada-Pace F, Senaratne P, Samadi DB (2011) Endorectal magnetic imaging has limited clinical ability to preoperatively predict pT3 prostate cancer. BJU Int 107:1419–1424
Wittekind C, Greene FL, Hutter RVP, Klimpfinger M, Sobin LH (2005) UICC: TNM classification of malignant tumours, 5th edn. Springer, Berlin Heidelberg
Beyersdorff D, Taymoorian K, Knösel T et al (2005) MRI of prostate cancer at 1.5 and 3.0T: comparision of image quality in tumor detection and staging. AJR Am J Roentgenol 185:1214–1220
Fütterer JJ, Heijmink SWTPJ, Scheenen TWJ et al (2006) Prostate cancer: local staging at 3-T endorectal MR imaging—early experience. Radiology 238:184–191
Tewari A, Peabody J, Sarle R et al (2002) Technique of the da Vinci robot-assissted anatomic radical prostatectomy. Urology 60:569–572
Srigley JR (2006) Key issues in handling and reporting radial prostatectomy specimens. Arch Pethol Lab Med 130:303–317
Epstein JI, Amin M, Boccon-Gibod L et al (2005) Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scan J Urol Nephrol Suppl 216:34–63
Sung M-T, Lin H, Koch MO, Davidson DD, Cheng L (2007) Radial distance of extraprostatic extension measured by ocular micrometer is an independent predictor of prostate-specific antigen recurrence. Am J Surg Pathol 31:311–318
Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL, Grading Committee ISUP (2005) The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Postatic carcinoma. Am J Surg Pathol 29:1228–1242
Bloch BN, Furman-Haran E, Helbich TH et al (2007) Prostate cancer: accurate determination of extracapsular extension with high-spatial-resolution dynamic contrast-enhanced and T2-weighted MR imaging—initial results. Radiology 245:176–185
Fütterer JJ, Engelbrecht MR, Huisman HJ et al (2005) Staging prostate cancer with dynamic contrast-enhanced endorectal MR imaging prior to radical prostatectomy: experienced versus less experienced readers. Radiology 237:541–554
Tan JS, Thng CH, Tan PH et al (2008) Local experience of endorectal magnetic resonance imaging of prostate with correlation to radical prostatectomy specimens. Ann Acad Med Singap 37:40–43
Nakashima J, Tanimoto A, Imai Y et al (2004) Endorectal MRI for prediction of tumor site, tumor size, and local extension of prostate cancer. Urology 64:101–105
Kim B, Breau RH, Papadatos D et al (2010) Diagnostic accuracy of surface coil magnetic resonance imaging at 1.5 T for local staging of elevated risk prostate cancer. Can Urol Assoc J 4:257–262
Magi-Galluzzi C, Evans AJ, Delahunt B, ISUP Prostate Cancer Group et al (2011) International society of urological pathology (ISUP) consensus conference on handling and staging radical prostatectomy specimens. Working group 3: extraprostatic extension, lymphovascular invasion and locally advanced disease. Mol Pathol 24:26–38
Egevad L, Srigley JR, Delahunt B, The ISUP Consensus Working Group (2011) International society of urological pathology consensus conference on handling and staging of radical prostatectomy specimens. Adv Anat Pathol 18:301–305
Balleyguier C, Sala E, Da Cunha T et al (2011) Staging of uterine cancer with MRI: guidelines of the European Society of Urogenital Radiology. Eur Radiol 21:1102–1110
Kinkel K, Forstner R, Danza FM et al (2009) Staging of endometrial cancer with MRI: guidelines of the European Society of Urogenital Imaging. Eur Radiol 19:1565–1574
Danneman D, Wiklund F, Wiklund P, Egevad L (2012) Prognostic significance of extraprostatic extension of prostate cancer. Avaliable at http://www.nature.com/labinvest/journal/v92/n1s/pdf/labinvest201235a.pdf?WT.ec_id=LABINVEST-201202. Accessed 3 April 2012
Gomez-Gelvez JC, Diaz-Insua M, Menon M, Gupta N (2012) Can quantitation and sub-categorization of extraprostatic extension (EPE) predict biochemical recurrence (BCR). Available at http://www.nature.com/labinvest/journal/v92/n1s/pdf/labinvest201235a.pdf?WT.ec_id=LABINVEST-201202. Accessed 3 April 2012
Acknowledgments
The authors wish to acknowledge Thorsten Feiweier (Siemens, Erlangen) for providing the DWI pulse sequence (research option). We gratefully acknowledge the financial support from the Norwegian Cancer Society (T.S.).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Hole, K.H., Axcrona, K., Lie, A.K. et al. Routine pelvic MRI using phased-array coil for detection of extraprostatic tumour extension: accuracy and clinical significance. Eur Radiol 23, 1158–1166 (2013). https://doi.org/10.1007/s00330-012-2669-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2669-x