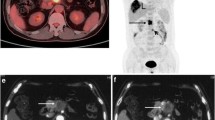
Abstract
Positron emission tomography (PET) has been used for the characterization of pancreatic and periampullary lesions. Pancreatitis-associated inflammation affecting only a portion of the pancreas gives the appearance of a mass lesion on imaging. Consequently, the differential diagnosis between cancer and pancreatitis becomes a commonly encountered problem. Traditionally, PET was interpreted as positive (to denote malignancy) if fluorodeoxyglucose (FDG) activity in the pancreas exceeded background activity and as negative (to denote benign) if activity was less than or equal to background activity. However, the specificity was limited with this method of interpretation. A relatively wide overlap has been reported between semiquantitative uptake values obtained in cancers and those in inflammatory lesions. Also, the qualitative (metabolic patterns) and quantitative variables (standardized uptake values) have been complementary and at sometimes controversial to each other in various clinical situations. There is paucity of data in the literature highlighting the role of FDG PET/CT in characterization of such mass lesions. The primary aim of this pictorial review is to list the various pathologic processes of pancreas and periampulla that could be studied with FDG PET/CT and recognize the different FDG uptake patterns and apply this information to characterize the different lesions affecting the pancreas and periampulla. We have also discussed the limitations of conventional imaging and advantages of FDG PET/CT for the evaluation mass-forming lesions of the pancreas and periampulla.




























Similar content being viewed by others
References
Pakzad F, Groves AM, Ell PJ (2006) The role of positron emission tomography in the management of pancreatic cancer. Semin Nucl Med 36(3):248–256. doi:10.1053/j.semnuclmed.2006.03.005
Wakabayashi H, Nishiyama Y, Otani T, et al. (2008) Role of 18F-fluorodeoxyglucose positron emission tomography imaging in surgery for pancreatic cancer. World J Gastroenterol 14(1):64–69
Delbeke D, Martin WH (2010) PET and PET/CT for pancreatic malignancies. Surg Oncol Clin N Am 19(2):235–254. doi:10.1016/j.soc.2009.11.005
Nichols MT, Russ PD, Chen YK (2006) Pancreatic imaging: current and emerging technologies. Pancreas 33(3):211–220. doi:10.1097/01.mpa.0000227912.71202.2c
Keogan MT, Tyler D, Clark L, et al. (1998) Diagnosis of pancreatic carcinoma: role of FDG PET. AJR Am J Roentgenol 171(6):1565–1570. doi:10.2214/ajr.171.6.9843289
Higashi T, Saga T, Nakamoto Y, et al. (2003) Diagnosis of pancreatic cancer using fluorine-18 fluorodeoxyglucose positron emission tomography (FDG PET)—usefulness and limitations in “clinical reality”. Ann Nucl Med 17(4):261–279
Zimny M, Schumpelick V (2001) Fluorodeoxyglucose positron emission tomography (FDG-PET) in the differential diagnosis of pancreatic lesions. Chirurg 72(9):989–994
Diederichs CG, Staib L, Vogel J, et al. (2000) Values and limitations of 18F-fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas 20(2):109–116
Delbeke D, Rose DM, Chapman WC, et al. (1999) Optimal interpretation of FDG PET in the diagnosis, staging and management of pancreatic carcinoma. J Nucl Med 40(11):1784–1791
Koyama K, Okamura T, Kawabe J, et al. (2001) Diagnostic usefulness of FDG PET for pancreatic mass lesions. Ann Nucl Med 15(3):217–224
Gambhir SS, Czernin J, Schwimmer J, et al. (2001) A tabulated summary of the FDG PET literature. J Nucl Med 42(5 Suppl):1S–93S
von Schulthess GK, Steinert HC, Hany TF (2006) Integrated PET/CT: current applications and future directions. Radiology 238(2):405–422. doi:10.1148/radiol.2382041977
Heinrich S, Goerres GW, Schafer M, et al. (2005) Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg 242(2):235–243
Kauhanen SP, Komar G, Seppanen MP, et al. (2009) A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 250(6):957–963. doi:10.1097/SLA.0b013e3181b2fafa
Schick V, Franzius C, Beyna T, et al. (2008) Diagnostic impact of 18F-FDG PET-CT evaluating solid pancreatic lesions versus endosonography, endoscopic retrograde cholangiopancreatography with intraductal ultrasonography and abdominal ultrasound. Eur J Nucl Med Mol Imaging 35(10):1775–1785. doi:10.1007/s00259-008-0818-x
Luttges J (2011) What’s new? The 2010 WHO classification for tumours of the pancreas. Pathologe 32(Suppl 2):332–336. doi:10.1007/s00292-011-1515-2
Bares R, Klever P, Hellwig D, et al. (1993) Pancreatic cancer detected by positron emission tomography with 18F-labelled deoxyglucose: method and first results. Nucl Med Commun 14(7):596–601
Friess H, Langhans J, Ebert M, et al. (1995) Diagnosis of pancreatic cancer by 2[18F]-fluoro-2-deoxy-d-glucose positron emission tomography. Gut 36(5):771–777
Santhosh S, Mittal BR, Bhasin D, et al. (2013) Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in the characterization of pancreatic masses: experience from tropics. J Gastroenterol Hepatol 28(2):255–261. doi:10.1111/jgh.12068
Riker A, Libutti SK, Bartlett DL (1997) Advances in the early detection, diagnosis, and staging of pancreatic cancer. Surg Oncol 6(3):157–169
Johnson PT, Outwater EK (1999) Pancreatic carcinoma versus chronic pancreatitis: dynamic MR imaging. Radiology 212(1):213–218
van Kouwen MC, Jansen JB, van Goor H, et al. (2005) FDG-PET is able to detect pancreatic carcinoma in chronic pancreatitis. Eur J Nucl Med Mol Imaging 32(4):399–404. doi:10.1007/s00259-004-1689-4
Rojas Y, Warneke CL, Dhamne CA, et al. (2012) Primary malignant pancreatic neoplasms in children and adolescents: a 20 year experience. J Pediatr Surg 47(12):2199–2204. doi:10.1016/j.jpedsurg.2012.09.005
Sperti C, Bissoli S, Pasquali C, et al. (2007) 18-fluorodeoxyglucose positron emission tomography enhances computed tomography diagnosis of malignant intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 246(6):932–937; discussion 937–939. doi:10.1097/SLA.0b013e31815c2a29
Hong HS, Yun M, Cho A, et al. (2010) The utility of F-18 FDG PET/CT in the evaluation of pancreatic intraductal papillary mucinous neoplasm. Clin Nucl Med 35(10):776–779. doi:10.1097/RLU.0b013e3181e4da32
Hara T, Ikebe D, Odaka A, et al. (2013) Preoperative Histological Subtype Classification of Intraductal Papillary Mucinous Neoplasms (IPMN) by Pancreatic Juice Cytology With MUC Stain. Ann Surg 257(6):1103–1111. doi:10.1097/SLA.0b013e318281b824
Sperti C, Pasquali C, Decet G, et al. (2005) F-18-fluorodeoxyglucose positron emission tomography in differentiating malignant from benign pancreatic cysts: a prospective study. J Gastrointest Surg 9(1):22–28; discussion 28–29. doi:10.1016/j.gassur.2004.10.002
Tann M, Sandrasegaran K, Jennings SG, et al. (2007) Positron-emission tomography and computed tomography of cystic pancreatic masses. Clin Radiol 62(8):745–751. doi:10.1016/j.crad.2007.01.023
Oberg K, Eriksson B (2005) Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol 19(5):753–781. doi:10.1016/j.bpg.2005.06.002
Kloppel G (2011) Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 18(Suppl 1):S1–S16. doi:10.1530/erc-11-0013
Kim HS, Joo SH, Yang DM, et al. (2011) Carcinosarcoma of the pancreas: a unique case with emphasis on metaplastic transformation and the presence of undifferentiated pleomorphic high-grade sarcoma. J Gastrointestin Liver Dis 20(2):197–200
Zhu WY, Liu TG, Zhu H (2012) Long-term recurrence-free survival in a patient with pancreatic carcinosarcoma: a case report with a literature review. Med Oncol (Northwood, London, England) 29(1):140–143. doi:10.1007/s12032-010-9804-9
Freeman C, Berg JW, Cutler SJ (1972) Occurrence and prognosis of extranodal lymphomas. Cancer 29(1):252–260
Ezzat A, Jamshed A, Khafaga Y, et al. (1996) Primary pancreatic non-Hodgkin’s lymphomas. J Clin Gastroenterol 23(2):109–112
Yoon SN, Lee MH, Yoon JK (2004) F-18 FDG positron emission tomography findings in primary pancreatic lymphoma. Clin Nucl Med 29(9):574–575
Salvatore JR, Cooper B, Shah I, et al. (2000) Primary pancreatic lymphoma: a case report, literature review, and proposal for nomenclature. Med Oncol (Northwood, London, England) 17(3):237–247
Kamisawa T, Egawa N, Nakajima H, et al. (2003) Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol 98(12):2694–2699. doi:10.1111/j.1572-0241.2003.08775.x
Nakazawa T, Ohara H, Sano H, et al. (2007) Difficulty in diagnosing autoimmune pancreatitis by imaging findings. Gastrointest Endosc 65(1):99–108. doi:10.1016/j.gie.2006.03.929
Kamisawa T, Funata N, Hayashi Y, et al. (2003) A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol 38(10):982–984. doi:10.1007/s00535-003-1175-y
Lee TY, Kim MH, Park do H, et al. (2009) Utility of 18F-FDG PET/CT for differentiation of autoimmune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer. AJR Am J Roentgenol 193(2):343–348. doi:10.2214/ajr.08.2297
Santhosh S, Bhattacharya A, Harisankar CN, Kochhar R, Mittal BR (2013) Role of 18F-FDG PET/CT in the Management of a Case of Autoimmune Pancreatitis With Extrapancreatic Manifestations. Clin Nucl Med . doi:10.1097/RLU.0b013e31827086b5
Sanabe N, Ikematsu Y, Nishiwaki Y, et al. (2002) Pancreatic tuberculosis. J Hepatobiliary Pancreat Surg 9(4):515–518. doi:10.1007/s005340200065
Nagar AM, Raut AA, Morani AC, et al. (2009) Pancreatic tuberculosis: a clinical and imaging review of 32 cases. J Comput Assist Tomogr 33(1):136–141. doi:10.1097/RCT.0b013e31816c82bc
Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas (2005). Gut 54(Suppl 5):v1–16. doi:10.1136/gut.2004.057059
Adsay V, Ohike N, Tajiri T, et al. (2012) Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol 36(11):1592–1608. doi:10.1097/PAS.0b013e31826399d8
Kalady MF, Clary BM, Clark LA, et al. (2002) Clinical utility of positron emission tomography in the diagnosis and management of periampullary neoplasms. Ann Surg Oncol 9(8):799–806
Author information
Authors and Affiliations
Corresponding author
Additional information
The data and work originated from the Postgraduate Institute of Medical Education and Research, Chandigarh (India). However, the corresponding author is currently working in the Tamil Nadu Government Multi Super Specialty Hospital, Chennai (India).
Rights and permissions
About this article
Cite this article
Santhosh, S., Mittal, B.R., Rana, S.S. et al. Metabolic signatures of malignant and non-malignant mass-forming lesions in the periampulla and pancreas in FDG PET/CT scan: an atlas with pathologic correlation. Abdom Imaging 40, 1285–1315 (2015). https://doi.org/10.1007/s00261-014-0266-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0266-y