Abstract
Purpose
Programmed cell death protein-1/ligand-1 (PD-1/L1) blockade has been a breakthrough in the treatment of patients with non-small cell lung cancer (NSCLC), but there is still a lack of effective methods to screen patients. Here we report a novel 68 Ga-labeled nanobody [68 Ga]Ga-THP-APN09 for PET imaging of PD-L1 status in mouse models and a first-in-human study in NSCLC patients.
Methods
[68 Ga]Ga-THP-APN09 was prepared by site-specific radiolabeling, with no further purification. Cell uptake assays were completed in the human lung adenocarcinoma cell line A549, NSCLC cell line H1975 and human PD-L1 gene-transfected A549 cells (A549PD−L1). The imaging to image PD-L1 status and biodistribution were investigated in tumor-bearing mice of these three tumor cell types. The first-in-human clinical translational trial was registered as NCT05156515. The safety, radiation dosimetry, biodistribution, and correlations of tracer uptake with immunohistochemical staining and major pathologic response (MPR) were evaluated in NSCLC patients who underwent adjuvant immunotherapy combined with chemotherapy.
Results
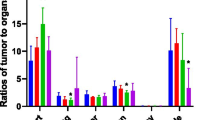
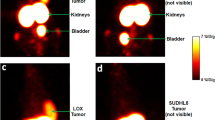
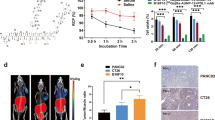
Radiosynthesis of [68 Ga]Ga-THP-APN09 was achieved at room temperature and a pH of 6.0–6.5 in 10 min with a high radiochemical yield (> 99%) and 13.9–27.8 GBq/μmol molar activity. The results of the cell uptake study reflected variable levels of surface PD-L1 expression observed by flow cytometry in the order A549PD−L1 > H1975 > A549. In small-animal PET/CT imaging, H1975 and A549PD−L1 tumors were clearly visualized in an 8.3:1 and 2.2:1 ratios over PD-L1-negative A549 tumors. Ex vivo biodistribution studies showed that tumor uptake was consistent with the PET results, with the highest A549PD−L1 being taken up the most (8.20 ± 0.87%ID/g), followed by H1975 (3.69 ± 0.50%ID/g) and A549 (0.90 ± 0.16%ID/g). Nine resectable NSCLC patients were enrolled in the clinical study. Uptake of [68 Ga]Ga-THP-APN09 was mainly observed in the kidneys and spleen, followed by low uptake in bone marrow. The radiation dose is within a reliable range. Tumor uptake was positively correlated with PD-L1 expression TPS (rs = 0.8763, P = 0.019). Tumor uptake of [68 Ga]Ga-THP-APN09 (SUVmax) in MPR patients was higher than that in non-MPR patients (median SUVmax 2.73 vs. 2.10, P = 0.036, determined with Mann–Whitney U-test).
Conclusion
[68 Ga]Ga-THP-APN09 has the potential to be transformed into a kit-based radiotracer for rapid, simple, one-step, room temperature radiolabeling. The tracer can detect PD-L1 expression levels in tumors, and it may make it possibility to predict the response of PD-1 immunotherapy combined with chemotherapy. Confirmation in a large number of cases is needed.
Trial registration
Clinical Trial (NCT05156515). Registered 12 December 2021.
Graphical abstract








Similar content being viewed by others
Data Availability
All data reported in this study can be found in the main manuscript or the supplementary materials. Correspondence and request for materials should be addressed to the corresponding authors.
References
Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. https://doi.org/10.1186/s40425-018-0316-z.
Kim TK, Vandsemb EN, Herbst RS, Chen L. Adaptive immune resistance at the tumour site: mechanisms and therapeutic opportunities. Nat Rev Drug Discovery. 2022;21:529–40. https://doi.org/10.1038/s41573-022-00493-5.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379:2342–50. https://doi.org/10.1056/NEJMoa1809697.
Vansteenkiste J, Wauters E, Reymen B, Ackermann CJ, Peters S, De Ruysscher D. Current status of immune checkpoint inhibition in early-stage NSCLC. Ann Oncol. 2019;30:1244–53. https://doi.org/10.1093/annonc/mdz175.
Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18:345–62. https://doi.org/10.1038/s41571-021-00473-5.
Lu S, Stein JE, Rimm DL, Wang DW, Bell JM, Johnson DB, et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA Oncol. 2019;5:1195–204. https://doi.org/10.1001/jamaoncol.2019.1549.
Hofman P. The challenges of evaluating predictive biomarkers using small biopsy tissue samples and liquid biopsies from non-small cell lung cancer patients. J Thorac Dis. 2019;11:S57-s64. https://doi.org/10.21037/jtd.2018.11.85.
Kluger HM, Zito CR, Turcu G, Baine MK, Zhang H, Adeniran A, et al. PD-L1 Studies Across Tumor Types, Its Differential Expression and Predictive Value in Patients Treated with Immune Checkpoint Inhibitors. Clin Cancer Res. 2017;23:4270–9. https://doi.org/10.1158/1078-0432.Ccr-16-3146.
Aguiar PN Jr, De Mello RA, Hall P, Tadokoro H, Lima LG. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy. 2017;9:499–506. https://doi.org/10.2217/imt-2016-0150.
Shukuya T, Carbone DP. Predictive Markers for the Efficacy of Anti-PD-1/PD-L1 Antibodies in Lung Cancer. J Thorac Oncol. 2016;11:976–88. https://doi.org/10.1016/j.jtho.2016.02.015.
Sacher AG, Gandhi L. Biomarkers for the Clinical Use of PD-1/PD-L1 Inhibitors in Non-Small-Cell Lung Cancer: A Review. JAMA Oncol. 2016;2:1217–22. https://doi.org/10.1001/jamaoncol.2016.0639.
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. The Lancet. 2017;389:255–65. https://doi.org/10.1016/s0140-6736(16)32517-x.
Bensch F, van der Veen EL, Lub-de Hooge MN, Jorritsma-Smit A, Boellaard R, Kok IC, et al. (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24:1852–8. https://doi.org/10.1038/s41591-018-0255-8.
Smit J, Borm FJ, Niemeijer AN, Huisman MC, Hoekstra OS, Boellaard R, et al. PD-L1 PET/CT Imaging with Radiolabeled Durvalumab in Patients with Advanced-Stage Non-Small Cell Lung Cancer. J Nucl Med. 2022;63:686–93. https://doi.org/10.2967/jnumed.121.262473.
Verhoeff SR, van de Donk PP, Aarntzen E, Oosting SF, Brouwers AH, Miedema IHC, et al. (89)Zr-DFO-Durvalumab PET/CT Before Durvalumab Treatment in Patients with Recurrent or Metastatic Head and Neck Cancer. J Nucl Med. 2022;63:1523–30. https://doi.org/10.2967/jnumed.121.263470.
Niemeijer AN, Leung D, Huisman MC, Bahce I, Hoekstra OS, van Dongen G, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun. 2018;9:4664. https://doi.org/10.1038/s41467-018-07131-y.
Huisman MC, Niemeijer AN, Windhorst AD, Schuit RC, Leung D, Hayes W, et al. Quantification of PD-L1 Expression with (18)F-BMS-986192 PET/CT in Patients with Advanced-Stage Non-Small Cell Lung Cancer. J Nucl Med. 2020;61:1455–60. https://doi.org/10.2967/jnumed.119.240895.
Nienhuis PH, Antunes IF, Glaudemans A, Jalving M, Leung D, Noordzij W, et al. (18)F-BMS986192 PET Imaging of PD-L1 in Metastatic Melanoma Patients with Brain Metastases Treated with Immune Checkpoint Inhibitors: A Pilot Study. J Nucl Med. 2022;63:899–905. https://doi.org/10.2967/jnumed.121.262368.
Zhou X, Jiang J, Yang X, Liu T, Ding J, Nimmagadda S, et al. First-in-Humans Evaluation of a PD-L1-Binding Peptide PET Radiotracer in Non-Small Cell Lung Cancer Patients. J Nucl Med. 2022;63:536–42. https://doi.org/10.2967/jnumed.121.262045.
Zhou M, Wang X, Chen B, Xiang S, Rao W, Zhang Z, et al. Preclinical and first-in-human evaluation of (18)F-labeled D-peptide antagonist for PD-L1 status imaging with PET. Eur J Nucl Med Mol Imaging. 2022;49:4312–24. https://doi.org/10.1007/s00259-022-05876-9.
Verhaar ER, Woodham AW, Ploegh HL. Nanobodies in cancer. Semin Immunol. 2021;52:101425. https://doi.org/10.1016/j.smim.2020.101425.
Wei W, Younis MH, Lan X, Liu J, Cai W. Single-Domain Antibody Theranostics on the Horizon. J Nucl Med. 2022;63:1475–9. https://doi.org/10.2967/jnumed.122.263907.
Yang EY, Shah K. Nanobodies: Next Generation of Cancer Diagnostics and Therapeutics. Front Oncol. 2020;10:1182. https://doi.org/10.3389/fonc.2020.01182.
Lv G, Sun X, Qiu L, Sun Y, Li K, Liu Q, et al. PET Imaging of Tumor PD-L1 Expression with a Highly Specific Nonblocking Single-Domain Antibody. J Nucl Med. 2020;61:117–22. https://doi.org/10.2967/jnumed.119.226712.
Qin X, Guo X, Liu T, Li L, Zhou N, Ma X, et al. High in-vivo stability in preclinical and first-in-human experiments with [(18)F]AlF-RESCA-MIRC213: a (18)F-labeled nanobody as PET radiotracer for diagnosis of HER2-positive cancers. Eur J Nucl Med Mol Imaging. 2023;50:302–13. https://doi.org/10.1007/s00259-022-05967-7.
Hu B, Liu T, Li L, Shi L, Yao M, Li C, et al. IgG-Binding Nanobody Capable of Prolonging Nanobody-Based Radiotracer Plasma Half-Life and Enhancing the Efficacy of Tumor-Targeted Radionuclide Therapy. Bioconjug Chem. 2022;33:1328–39. https://doi.org/10.1021/acs.bioconjchem.2c00209.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. https://doi.org/10.1007/s00259-014-2961-x.
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378:2288–301. https://doi.org/10.1056/NEJMoa1716948.
Ilie M, Long-Mira E, Bence C, Butori C, Lassalle S, Bouhlel L, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. 2016;27:147–53. https://doi.org/10.1093/annonc/mdv489.
Mayer AT, Natarajan A, Gordon SR, Maute RL, McCracken MN, Ring AM, et al. Practical Immuno-PET Radiotracer Design Considerations for Human Immune Checkpoint Imaging. J Nucl Med. 2017;58:538–46. https://doi.org/10.2967/jnumed.116.177659.
Young JD, Abbate V, Imberti C, Meszaros LK, Ma MT, Terry SYA, et al. (68)Ga-THP-PSMA: A PET Imaging Agent for Prostate Cancer Offering Rapid, Room-Temperature, 1-Step Kit-Based Radiolabeling. J Nucl Med. 2017;58:1270–7. https://doi.org/10.2967/jnumed.117.191882.
Derlin T, Schmuck S, Juhl C, Zorgiebel J, Schneefeld SM, Walte ACA, et al. PSA-stratified detection rates for [(68)Ga]THP-PSMA, a novel probe for rapid kit-based (68)Ga-labeling and PET imaging, in patients with biochemical recurrence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:913–22. https://doi.org/10.1007/s00259-017-3924-9.
Donnelly DJ, Smith RA, Morin P, Lipovsek D, Gokemeijer J, Cohen D, et al. Synthesis and Biologic Evaluation of a Novel (18)F-Labeled Adnectin as a PET Radioligand for Imaging PD-L1 Expression. J Nucl Med. 2018;59:529–35. https://doi.org/10.2967/jnumed.117.199596.
Crauwels M, Massa S, Martin C, Betti C, Ballet S, Devoogdt N, et al. Site-Specific Radioactive Labeling of Nanobodies. Methods Mol Biol (Clifton, NJ). 2018;1827:505–40. https://doi.org/10.1007/978-1-4939-8648-4_26.
Satpati D, Shinto A, Kamaleshwaran KK, Sane S, Banerjee S. Convenient Preparation of [(68)Ga]DKFZ-PSMA-11 Using a Robust Single-Vial Kit and Demonstration of Its Clinical Efficacy. Mol Imaging Biol. 2016;18:420–7. https://doi.org/10.1007/s11307-016-0943-z.
Altunay B, Goedicke A, Winz OH, Hertel F, von Mallek D, Meszaros LK, et al. (99m)Tc-labeled single-domain antibody for SPECT/CT assessment of HER2 expression in diverse cancer types. Eur J Nucl Med Mol Imaging. 2023;50:1005–13. https://doi.org/10.1007/s00259-022-06066-3.
Altunay B, Morgenroth A, Beheshti M, Vogg A, Wong NCL, Ting HH, et al. HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging. Eur J Nucl Med Mol Imaging. 2021;48:1371–89. https://doi.org/10.1007/s00259-020-05094-1.
Behr TM, Goldenberg DM, Becker W. Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: present status, future prospects and limitations. Eur J Nucl Med. 1998;25:201–12. https://doi.org/10.1007/s002590050216.
Chen X, Gao A, Zhang F, Yang Z, Wang S, Fang Y, et al. ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation. Theranostics. 2021;11:3392–416. https://doi.org/10.7150/thno.52435.
Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90. https://doi.org/10.1084/jem.20131916.
Tracy SI, Venkatesh H, Hekim C, Heltemes-Harris LM, Knutson TP, Bachanova V, et al. Combining nilotinib and PD-L1 blockade reverses CD4+ T-cell dysfunction and prevents relapse in acute B-cell leukemia. Blood. 2022;140:335–48. https://doi.org/10.1182/blood.2021015341.
Fricke J, Mambetsariev I, Pharaon R, Subbiah S, Rajurkar S, Salgia R. Hyperprogression on immunotherapy with complete response to chemotherapy in a NSCLC patient with high PD-L1 and STK11: A case report. Medicine (Baltimore). 2020;99:e22323. https://doi.org/10.1097/md.0000000000022323.
Acknowledgements
The authors would like to thank Dr. Huifang Tian, Key Laboratory of Carcinogenesis and Translational Research, (Ministry of Education), Central Laboratory, Peking University Cancer Hospital & Institute (Beijing, China) for her technical assistance in MALDI analysis.
Funding
This research was supported by grants from the National Natural Science Foundation of China, grant number (No. 81871416, 82172006); the Capital’s Funds for Health Improvement and Research (No. 2022-2Z-2154 and 2022-2Z-2155); the Beijing Millions of Talent Projects A level funding, grant number (No. 2019A38); the Beijing Hospitals Authority Deng feng Project (DFL20191102); the Beijing Municipal Administration of Hospitals Yang fan Project (ZYLX201816); the Pilot Project (4th Round) to Reform Public Development of Beijing Municipal Medical Research Institute (2021–1).
Author information
Authors and Affiliations
Contributions
NL, HZ, BJ and ZY conceived and designed this research. XM was responsible for all experiments, data collection and analysis, and wrote the manuscript. XZ was responsible for the recruitment of patients and image analysis. BH was responsible for screening and identification of the nanobody. XL, MY, LL, and XQ were involved in the preparation of radiopharmaceuticals. DL, YY, XH, SL, YC, and ZW took part in some animal experiments. WZ was involved in immunohistochemical experiments on animal tumors.
Corresponding authors
Ethics declarations
Ethics approval
All clinical studies were approved by the Medical Ethics Committee of Beijing Cancer Hospital (2021KT111), and registered in the Clinical Trial (NCT05156515). All animal studies were performed according to the guidelines of the Animal Care and Use Committee of Perking University Cancer Hospital.
Consent to participate
Written informed consent was obtained from all participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, X., Zhou, X., Hu, B. et al. Preclinical evaluation and pilot clinical study of [68Ga]Ga-THP-APN09, a novel PD-L1 targeted nanobody radiotracer for rapid one-step radiolabeling and PET imaging. Eur J Nucl Med Mol Imaging 50, 3838–3850 (2023). https://doi.org/10.1007/s00259-023-06373-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06373-3