Abstract
Purpose
To assess and compare the diagnostic accuracy of whole-body (WB) DW-MRI with 2-[18F]FDG PET for staging and treatment monitoring of children with Langerhans cell histiocytosis (LCH).
Methods
Twenty-three children with LCH underwent 2-[18F]FDG PET and WB DW-MRI at baseline. Two nuclear medicine physicians and two radiologists independently assessed presence/absence of tumors in 8 anatomical areas. Sixteen children also performed 2-[18F]FDG PET and WB DW-MRI at follow-up. One radiologist and one nuclear medicine physician revised follow-up scans and collected changes in tumor apparent diffusion (ADC) and standardized uptake values (SUV) before and after therapy in all detectable lesions. 2-[18F]FDG PET results were considered the standard of reference for tumor detection and evaluation of treatment response according to Lugano criteria. Sensitivity, specificity, positive and negative predictive values, and diagnostic accuracy of WB DW-MRI at baseline were calculated, and the 95% confidence intervals were estimated by using the Clopper-Pearson (exact) method; changes in tumor SUVs and ADC were compared using a Mann–Whitney U test. Agreement between reviewers was assessed with a Cohen’s weighted kappa coefficient. Analyses were conducted using SAS software version 9.4.
Results
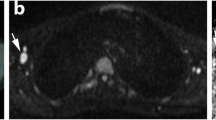
Agreement between reviewers was perfect (kappa coefficient = 1) for all analyzed regions but spine and neck (kappa coefficient = 0.89 and 0.83, respectively) for 2-[18F]FDG PET images, and abdomen and pelvis (kappa coefficient = 0.65 and 0.88, respectively) for WB DW-MRI. Sensitivity and specificity were 95.5% and 100% for WB DW-MRI compared to 2-[18F]FDG PET. Pre to post-treatment changes in SUVratio and ADCmean were inversely correlated for all lesions (r: -0.27, p = 0·06) and significantly different between responders and non-responders to chemotherapy (p = 0.0006 and p = 0·003 for SUVratio and ADCmean, respectively).
Conclusion
Our study showed that WB DW-MRI has similar accuracy to 2-[18F]FDG PET for staging and treatment monitoring of LCH in children. While 2-[18F]FDG PET remains an approved radiological examination for assessing metabolically active disease, WB DW-MRI could be considered as an alternative approach without radiation exposure. The combination of both modalities might have advantages over either approach alone.





Similar content being viewed by others
Data availability
All data generated or analyzed during the study are included in the published paper.
References
Stalemark H, Laurencikas E, Karis J, Gavhed D, Fadeel B, Henter JI. Incidence of Langerhans cell histiocytosis in children: a population-based study. Pediatr Blood Cancer. 2008;51:76–81.
Alston RD, Tatevossian RG, McNally RJ, Kelsey A, Birch JM, Eden TO. Incidence and survival of childhood Langerhans cell histiocytosis in Northwest England from 1954 to 1998. Pediatr Blood Cancer. 2007;48:555–60.
The WHO Classification of Tumours Editorial Board. WHO Classification of tumours soft tissue and bone tumours, 5th ed. Lyon: IARC Press; 2020.
Rodriguez-Galindo C, Allen CE. Langerhans cell histiocytosis. Blood. 2020;135:1319–31.
Kilpatrick SE, Wenger DE, Gilchrist GS, Shives TC, Wollan PC, Unni KK. Langerhans' cell histiocytosis (histiocytosis X) of bone. A clinicopathologic analysis of 263 pediatric and adult cases. Cancer. 1995;76:2471–2484.
Gadner H, Minkov M, Grois N, et al. Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood. 2013;121:5006–14.
Titgemeyer C, Grois N, Minkov M, Flucher-Wolfram B, Gatterer-Menz I, Gadner H. Pattern and course of single-system disease in Langerhans cell histiocytosis data from the DAL-HX 83- and 90-study. Med Pediatr Oncol. 2001;37:108–14.
Gadner H, Grois N, Arico M, et al. A randomized trial of treatment for multisystem Langerhans’ cell histiocytosis. J Pediatr. 2001;138:728–34.
Gadner H, Grois N, Potschger U, et al. Improved outcome in multisystem Langerhans cell histiocytosis is associated with therapy intensification. Blood. 2008;111:2556–62.
Milen Minkov NG, Kenneth McClain, et al. Langherans cell histiocytosis; Histiocyte Society; evaluation and treatment guidelines. http://www.hematologie-amc.nl/bestanden/hematologie/bijlagennietinDBS/SocietyLCHTreatmentGuidelines.PDF. 2009.
Baratto L, Hawk KE, States L, et al. PET/MRI improves management of children with cancer. J Nucl Med. 2021;62:1334–40.
Steinborn M, Wortler K, Nathrath M, Schoniger M, Hahn H. Rummeny EJ [Whole-body MRI in children with Langerhans cell histiocytosis for the evaluation of the skeletal system]. Rofo. 2008;180:646–53.
Kim JR, Yoon HM, Jung AY, Cho YA, Seo JJ, Lee JS. Comparison of whole-body MRI, bone scan, and radiographic skeletal survey for lesion detection and risk stratification of Langerhans cell histiocytosis. Sci Rep. 2019;9:317.
Lee HJ, Ahn BC, Lee SW, Lee J. The usefulness of F-18 fluorodeoxyglucose positron emission tomography/computed tomography in patients with Langerhans cell histiocytosis. Ann Nucl Med. 2012;26:730–7.
Albano D, Bosio G, Giubbini R, Bertagna F. Role of (18)F-FDG PET/CT in patients affected by Langerhans cell histiocytosis. Jpn J Radiol. 2017;35:574–83.
Rashidi A, Baratto L, Theruvath AJ, et al. Diagnostic accuracy of 2-[(18)F]FDG-PET and whole-body DW-MRI for the detection of bone marrow metastases in children and young adults. Eur Radiol. 2022.
Klenk C, Gawande R, Uslu L, et al. Ionising radiation-free whole-body MRI versus (18)F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: a prospective, non-randomised, single-centre study. Lancet Oncol. 2014;15:275–85.
Theruvath AJ, Siedek F, Muehe AM, et al. Therapy response assessment of pediatric tumors with whole-body diffusion-weighted MRI and FDG PET/MRI. Radiology. 2020;296:143–51.
Uslu-Besli L, AtayKapucu LO, Karadeniz C, et al. Comparison of FDG PET/MRI and FDG PET/CT in pediatric oncology in terms of anatomic correlation of FDG-positive lesions. J Pediatr Hematol Oncol. 2019;41:542–50.
Niu J, Liang J, Feng Q, et al. (18)F-FDG PET/MR assessment of pediatric Langerhans cell histiocytosis. Int J Gen Med. 2021;14:6251–9.
Surov A, Meyer HJ, Wienke A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: a meta-analysis. Oncotarget. 2017;8:59492–9.
Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Wu X, Kellokumpu-Lehtinen PL, Pertovaara H, et al. Diffusion-weighted MRI in early chemotherapy response evaluation of patients with diffuse large B cell lymphoma–a pilot study: comparison with 2-deoxy-2-fluoro- D-glucose-positron emission tomography/computed tomography. NMR Biomed. 2011;24:1181–90.
Phillips M, Allen C, Gerson P, McClain K. Comparison of FDG-PET scans to conventional radiography and bone scans in management of Langerhans cell histiocytosis. Pediatr Blood Cancer. 2009;52:97–101.
Kharuzhyk S, Zhavrid E, Dziuban A, Sukolinskaja E, Kalenik O. Comparison of whole-body MRI with diffusion-weighted imaging and PET/CT in lymphoma staging. Eur Radiol. 2020;30:3915–23.
Soydan L, Demir AA, Torun M, Cikrikcioglu MA. Use of diffusion-weighted magnetic resonance imaging and apparent diffusion coefficient in gastric cancer staging. Curr Med Imaging. 2020;16:1278–89.
Regacini R, Puchnick A, Luisi FAV, Lederman HM. Can diffusion-weighted whole-body MRI replace contrast-enhanced CT for initial staging of Hodgkin lymphoma in children and adolescents? Pediatr Radiol. 2018;48:638–47.
Le Bihan D, Poupon C, Amadon A, Lethimonnier F. Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging. 2006;24:478–88.
Schafer JF, Gatidis S, Schmidt H, et al. Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology. 2014;273:220–31.
Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175–84.
Funding
We acknowledge funding by the following NIH grants: R01CA26923 and P30CA124435 from the National Cancer Institute (NCI). Mariam Aboian was supported by KL2 TR001862 from the National Center for Advancing Translational Science (NCATS).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. HDL, AJT, RN, and LB wrote the manuscript. All authors reviewed and approved the final version of the manuscript. HDL, LB, AJT, AS, and KEH performed data collection, analysis, and interpretation. SS performed the statistical analysis. LB, MJ, and RN contributed to the literature search. LB, AR, and HDL are responsible for the figures. LS and MA shared important cases from their Institutions. HDL led the involvement of different pediatric Hospitals among the USA.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baratto, L., Nyalakonda, R., Theruvath, A.J. et al. Comparison of whole-body DW-MRI with 2-[18F]FDG PET for staging and treatment monitoring of children with Langerhans cell histiocytosis. Eur J Nucl Med Mol Imaging 50, 1689–1698 (2023). https://doi.org/10.1007/s00259-023-06122-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06122-6