Abstract
Purpose
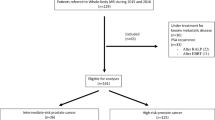
The aim of the study was to elaborate the incidence and type of skeletal involvement in a large cohort of patients with newly diagnosed prostate cancer (PCa) referred for Ga-68 PSMA-11 PET/CT staging in a single center.
Methods
Study cohort included 963 consecutive patients with newly diagnosed PCa referred for Ga-68 PSMA-11 PET/CT study for staging. The incidence of bone involvement, type of bone metastases, and extent of disease were determined and correlated with the ISUP Grade Group (GG) criteria and PSA levels.
Results
Bone metastases were found in 188 (19.5%) of 963 patients. Bone metastases were found in 10.7% of patients with PSA < 10 ng/dL and in 27.4% of patients with PSA > 10 ng/dL and in 6.1% of patients with GG ≤ 2/3 and in 8.9% of patients with GG 4/5. In 7.6% of the patients, skeletal involvement was extensive, while 11.9% of patients had oligometastatic disease. Osteoblastic type metastases were the most common type of bone metastases presented in 133 of the patients with malignant bone involvement (70.7%). More than half of them had only osteoblastic lesions (72 patients (38.3%)), while the other (61 patients (32.5%)) had also intramedullary and/or osteolytic type lesions. Intramedullary metastases were found in 97 patients (51.6%), while 41 (21.8%) of them were only intramedullary lesions. Osteolytic metastases were detected in 36 patients (19.2%), of which 8 were only osteolytic lesions.
Conclusion
Although traditionally bone metastases of PCa are considered osteoblastic, osteolytic and intramedullary metastases are common, as identified on PET with labeled PSMA. Skeletal spread may be present also in patients with GG ≤ 2/3 and PSA < 10 ng/dL.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Bertoldo F, Boccardo F, Bombardieri E, Evangelista L, Valdagni R. Bone metastases from prostate cancer. Biology, Diagnosis and Management. 2017; Springer International Publishing, Switzerland. https://doi.org/10.1007/978-3-319-42327-2.
Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–83. https://doi.org/10.1053/hp.2000.6698.
Nørgaard M, Jensen A, Jacobsen JB, et al. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol. 2010;184:162–7. https://doi.org/10.1016/j.juro.2010.03.034.
Donswijk ML, van Leeuwen PJ, Vegt E, et al. Clinical impact of PSMA PET/CT in primary prostate cancer compared to conventional nodal and distant staging: a retrospective single center study. BMC Cancer. 2020;20:723. https://doi.org/10.1186/s12885-020-07192-7.
Sachpekidis C, Bäumer P, Kopka K, et al. 68 Ga-PSMA PET/CT in the evaluation of bone metastases in prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45(6):904–12. https://doi.org/10.1007/s00259-018-3936-0.
Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol. 2018;199:683–90. https://doi.org/10.1016/j.juro.2017.11.095.
Donohoe KJ, Cohen EJ, Giammarile F, Grady E, Greenspan BS, Henkin RE, Millstine J, Smith GT, Srinivas S, Kauffman J, Ahuja S. Appropriate use criteria for bone scintigraphy in prostate and breast cancer: summary and excerpts. J Nucl Med. 2017;58(4):14N-17N (Newsline).
Coakley FV, Oto A, Expert Panel on Urologic Imaging, et al. ACR Appropriateness Criteria® prostate cancer-pretreatment detection, surveillance, and staging. J Am Coll Radiol. 2017;14(suppl):S245–57. https://doi.org/10.1016/j.jacr.2017.02.026.
Carroll PH, Mohler JL. NCCN guidelines updates: prostate cancer and prostate cancer early detection. J Natl Compr Canc Netw. 2018;16:620–3. https://doi.org/10.6004/jnccn.2018.0036.
Castellucci P, Ceci F, Fanti S. Imaging of prostate cancer using 11 C-choline PET/computed tomography. PET Clin. 2017;12(2):137–43. https://doi.org/10.1016/j.cpet.2016.11.002.
Beheshti M, Vali R, Waldenberger P, et al. Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and 18F fluoride PET-CT: a comparative study. Eur J Nucl Med Mol Imaging. 2008;35(10):1766–74. https://doi.org/10.1007/s00259-008-0788-z.
Kuten J, Mabjeesh NJ, Lerman H, et al. Ga-PSMA PET/CT staging of newly diagnosed intermediate- and high-risk prostate cancer. Isr Med Assoc J. 2019;21(2):100–4.
Zacho HD, Ravn S, Afshar-Oromieh A, et al. Added value of 68 Ga-PSMA PET/CT for the detection of bone metastases in patients with newly diagnosed prostate cancer and a previous 99m Tc bone scintigraphy. EJNMMI Res. 2020;10(1):31. https://doi.org/10.1186/s13550-020-00618-0.
Mcarthur C, Mclaughlin G, Meddings RN. Changing the referral criteria for bone scan in newly diagnosed prostate cancer patients. Br J Radiol. 2012;85(1012):390–4. https://doi.org/10.1259/bjr/79184355.
Corfield J, Perera M, Bolton D, et al. (68)Ga-prostate specific membrane antigen (PSMA) positron emission tomography (PET) for primary staging of high-risk prostate cancer: a systematic review. World J Urol. 2018;36:519–27. https://doi.org/10.1007/s00345-018-2182-1.
Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet. 2020;395:1208–16. https://doi.org/10.1016/S0140-6736(20)30314-7.
Zhou J, Gou Z, Wu R, et al. Comparison of PSMA-PET/CT, choline-PET/CT, NaF-PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a systematic review and meta-analysis. Skeletal Radiol. 2019;48:1915–24. https://doi.org/10.1007/s00256-019-03230-z.
Janssen JC, Woythal N, Meißner S, et al. [68 Ga]PSMA-HBED-CC Uptake in osteolytic, osteoblastic, and bone marrow metastases of prostate cancer patients. Mol Imaging Biol. 2017;19(6):933–43. https://doi.org/10.1007/s11307-017-1101-y.
van Leenders GJLH, van der Kwast TH, Grignon DJ, et al. The 2019 International Society of Urological Pathology (ISUP) consensus conference on grading of prostatic carcinoma. Am J Surg Pathol. 2020;44(8):e87–99. https://doi.org/10.1097/PAS.0000000000001497.
Rauscher I, Maurer T, Fendler WP, Sommer WH, Schwaiger M, Eiber M. 68Ga-PSMA ligand PET/CT in patients with prostate cancer: how we review and report. Cancer Imaging. 2016;16:1–10. https://doi.org/10.1186/s40644-016-0072-6.
Rowe SP, Pienta KJ, Pomper MG, et al. PSMA-RADS version 1.0: a step towards standardizing the interpretation and reporting of PSMA-targeted PET imaging studies. Eur Urol. 2018;73(4):485–7. https://doi.org/10.1016/j.eururo.2017.10.027.
Kuten J, Dekalo S, Mintz I, et al. The significance of equivocal bone findings in staging PSMA imaging in the preoperative setting: validation of the PSMA-RADS version 1.0. EJNMMI Res. 2021;11(1):3. https://doi.org/10.1186/s13550-020-00745-8.
Singh D, Yi WS, Brasacchio RA, Muhs AG, Smudzin T, Williams JP, Messing E, Okunieff P. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol Biol Phys. 2004;58:3–10. https://doi.org/10.1016/s0360-3016(03)01442-1.
Chen MY, Franklin A, Yaxley J. Solitary rib lesions showing prostate-specific membrane antigen (PSMA) uptake in pre-treatment staging 68 Ga-PSMA-11 positron emission tomography scans for men with prostate cancer: benign or malignant? BJU Int. 2020;126(3):396–401. https://doi.org/10.1111/bju.
Even-Sapir E. Imaging of malignant bone involvement by morphologic, scintigraphic, and hybrid modalities. J Nucl Med. 2005;46(8):1356–67.
Høilund-Carlsen PF, Hess S, Werner TJ, et al. Cancer metastasizes to the bone marrow and not to the bone: time for a paradigm shift! Eur J Nucl Med Mol Imaging. 2018;45(6):893–7. https://doi.org/10.1007/s00259-018-3959-6.
Larson SR, Zhang X, Dumpit R, et al. Characterization of osteoblastic and osteolytic proteins in prostate cancer bone metastases. Prostate. 2013;73(9):932–40. https://doi.org/10.1002/pros.22639.
Zacho HD, Barsi T, Mortensen JC, et al. Prospective multicenter study of bone scintigraphy in consecutive patients with newly diagnosed prostate cancer. Clin Nucl Med. 2014;39:26–31. https://doi.org/10.1097/RLU.0000000000000291.
Gupta M, Choudhury PS, Rawal S, et al. Initial risk stratification and staging in prostate cancer with prostatic-specific membrane antigen positron emission tomography/computed tomography: a first-stop-shop. World J Nucl Med. 2018;17(4):261–9. https://doi.org/10.4103/wjnm.WJNM_79_17.
Wondergem M, van der Zant FM, Broos WAM, et al. 18F-DCFPyL PET/CT for primary staging in 160 high-risk prostate cancer patients; metastasis detection rate, influence on clinical management and preliminary results of treatment efficacy. Eur J Nucl Med Mol Imaging. 2021;48(2):521–31. https://doi.org/10.1007/s00259-020-04782-2.
Klingenberg S, Jochumsen MR, Ulhøi BP, et al. 68Ga-PSMA PET/CT for primary lymph node and distant metastasis NM staging of high-risk prostate cancer. J Nucl Med. 2021;62(2):214–20. https://doi.org/10.2967/jnumed.120.245605.
Lengana T, Lawal IO, Boshomane TG, et al. 68Ga-PSMA PET/CT replacing bone scan in the initial staging of skeletal metastasis in prostate cancer: a fait accompli? Clin Genitourin Cancer. 2018;16(5):392–401. https://doi.org/10.1016/j.clgc.2018.07.009.
Ceci F, Castellucci P, Graziani T, et al. 11C-choline PET/CT identifies osteoblastic and osteolytic lesions in patients with metastatic prostate cancer. Clin Nucl Med. 2015;40:e265–70. https://doi.org/10.1097/RLU.0000000000000783.
Gauvin S, Rompré-Brodeur A, Chaussé G, et al. 18F-fluorocholine positron emission tomography-computed tomography (18F-FCH PET/CT) for staging of high-risk prostate cancer patients. Can Urol Assoc J. 2019;13(4):84–91. https://doi.org/10.5489/cuaj.5142.
de Galiza Barbosa F, Queiroz MA, Nunes RF et al. Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging. 2020;20:23. https://doi.org/10.1186/s40644-020-00300-7.
Author information
Authors and Affiliations
Contributions
MK, OY, and EES contributed to the conception of the study, data collection, study design, writing, and revising the manuscript; KK, ID, JK, CL, DS, and DK contributed to the data collection, writing, and revising the manuscript; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with human participants or animals performed by any of the authors. The study protocol was approved by the local institutional ethics committee (reference ID TLV-0327–20). All patients gave written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology—Genitourinary.
Rights and permissions
About this article
Cite this article
Kesler, M., Kerzhner, K., Druckmann, I. et al. Staging 68 Ga-PSMA PET/CT in 963 consecutive patients with newly diagnosed prostate cancer: incidence and characterization of skeletal involvement. Eur J Nucl Med Mol Imaging 49, 2077–2085 (2022). https://doi.org/10.1007/s00259-021-05655-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05655-y