Abstract
Purpose
The formation of advanced plaques, which is characterized by the uninterrupted aggregation of macrophages with high expression of folate receptor-β (FR-β), is observed in several concomitant metabolic syndromes. The objective of this study was to develop a novel FR-β-targeted single-photon emission computed tomography (SPECT) radiotracer and validate its application to the noninvasive detection of atherosclerosis (AS) plaque and non-alcoholic fatty liver (NAFL).
Methods
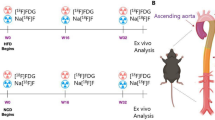
Two radioiodinated probes, [131I]IPBF and [131I]IBF, were developed, and cell uptake studies were used to identify their specific targets for activated macrophages. Biodistribution in normal mice was performed to obtain the pharmacokinetic information of the probes. Apolipoprotein E knockout (ApoE−/−) mice with atherosclerotic aortas were induced by a high-fat and high-cholesterol (HFHC) diet. To investigate the affinity of radiotracers to FR-β, Kd values were determined using in vitro assays. In addition, the assessments of the aorta in the ApoE−/− mice at different stages were performed using in vivo SPECT/CT imaging, and the findings were compared by histology.
Results
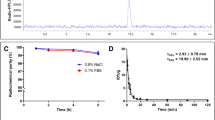
Both [131I]IPBF and [131I]IBF were synthesized with > 95% radiochemical purity and up to 3 MBq/nmol molar activity. In vitro assay of [131I]IPBF showed a moderate binding affinity to plasma proteins and specific uptake in activated macrophages. The prolonged blood elimination half-life (t1/2z) of [131I]IPBF (8.14 h) was observed in a pharmacokinetic study of normal mice, which was significantly longer than that of [131I]IBF (t1/2z = 2.95 h). As expected, the Kd values of [131I]IPBF and [131I]IBF in the Raw 264.7 cells were 43.94 ± 9.83 nM and 61.69 ± 15.19 nM, respectively. SPECT imaging with [131I]IPBF showed a high uptake in advanced plaques and NAFL. Radioactivity in excised aortas examined by ex vivo autoradiography further confirmed the specific uptake of [131I]IPBF in high-risk AS plaques.
Conclusions
In summary, we reported a proof-of-concept study of an albumin-binding folate derivative for macrophage imaging. The FR-β-targeted probe, [131I]IPBF, significantly prolongs the plasma elimination half-life and has the potential for the monitoring of AS plaques and concomitant fatty liver.







Similar content being viewed by others
Abbreviations
- AS:
-
Atherosclerosis
- FR-β:
-
Folate receptor-β
- NAFL:
-
Non-alcoholic fatty liver
- PET:
-
Positron emission tomography
- SPECT:
-
Single-photon emission computed tomography
- CVD:
-
Cardiovascular disease
- HSA:
-
Human serum albumin
- HPLC:
-
High-performance liquid chromatography
- Ox-LDL:
-
Oxidized-LDL
- ORO:
-
Oil red O
- FA:
-
Folic acid
- MWCO:
-
Molar weight cutoff
- WT:
-
Wild type
- HFHC:
-
High fat and high cholesterol
- ApoE−/− :
-
Apolipoprotein E knockout
- SMA:
-
Smooth muscle actin
- %IA/g:
-
Percentage of injected activity per gram
- ROIs:
-
Regions of interest
- H&E:
-
Hematoxylin-eosin
References
Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25.
Shaw L, Chandrashekhar Y. Progress in cardiovascular imaging. JACC Cardiovasc Imag. 2019;12:2589–610.
Nakahara T, Dweck MR, Narula N, Pisapia D, Narula J, Strauss HW. Coronary artery calcification: from mechanism to molecular imaging. JACC Cardiovasc Imag. 2017;10:582–93.
Lee SB, Park GM, Lee JY, Lee BU, Park JH, Kim BG, et al. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: an observational cohort study. J Hepatol. 2018;68:1018–24.
Sinn DH, Kang D, Chang Y, Ryu S, Gu S, Kim H, et al. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut. 2017;66:323–9.
Sinn DH, Cho SJ, Gu S, Seong D, Kang D, Kim H, et al. Persistent nonalcoholic fatty liver disease increases risk for carotid atherosclerosis. Gastroenterology. 2017;66:323–9.
Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, et al. Bidirectional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66:390–7.
Makowski MR, Wiethoff AJ, Blume U, Cuello F, Warley A, Jansen CHP, et al. Assessment of atherosclerotic plaque burden with an elastin-specific magnetic resonance contrast agent. Nat Med. 2011;17:383–8.
Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14:459–65.
Abele JT, Fung CI. Effect of hepatic steatosis on liver FDG uptake measured in mean standard uptake values. Radiology. 2010;254:917–24.
Moon SH, Hong SP, Cho YS, Noh TS, Choi JY, Kim BT, et al. Hepatic FDG uptake is associated with future cardiovascular events in asymptomatic individuals with non-alcoholic fatty liver disease. J Nucl Cardiol. 2015;24:892–9.
Lee HJ, Lee CH, Kim S, Hwang SY, Hong HC, Choi HY, et al. Association between vascular inflammation and non-alcoholic fatty liver disease: analysis by 18F-fluorodeoxyglucose positron emission tomography. Metabolism. 2017;67:72–9.
Daghem M, Bing R, Fayad ZA, Dweck MR. Noninvasive imaging to assess atherosclerotic plaque composition and disease activity: coronary and carotid applications. JACC Cardiovasc Imag. 2020;13:1055–68.
Tabas I, Bornfeldt KE. Intracellular and intercellular aspects of macrophage immunometabolism in atherosclerosis. Circ Res. 2020;126:1209–27.
Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145–59.
Park JW, Jeong G, Kim SJ, Kim MK, Park SM. Predictors reflecting the pathological severity of non-alcoholic fatty liver disease: comprehensive study of clinical and immunohistochemical findings in younger Asian patients. J Gastroenterol Hepatol. 2007;22:491–7.
Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? Int J Cancer. 2006;119:243–50.
Shi H, Guo J, Li C, Wang Z. A current review of folate receptor alpha as a potential tumor target in non-small-cell lung cancer. Drug Des Devel Ther. 2015;9:4989–96.
O’Shannessy DJ, Somers EB, Maltzman J, Smale R, Fu YS. Folate receptor alpha (FRA) expression in breast cancer: identification of a new molecular subtype and association with triple negative disease. Springerplus. 2012;1:22.
Jager NA, Westra J, Golestani R, van Dam GM, Low PS, Tio RA, et al. Folate receptor-β imaging using 99mTc-folate to explore distribution of polarized macrophage populations in human atherosclerotic plaque. J Nucl Med. 2014;55:1945–51.
Müller A, Beck K, Rancic Z, Müller C, Fischer CR, Betzel T, et al. Imaging atherosclerotic plaque inflammation via folate receptor targeting using a novel 18F-folate radiotracer. Mol Imaging. 2014;13:1–11.
Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113:438–46.
Williams JW, Giannarelli C, Rahman A, Randolph GJ, Kovacic JC. Macrophage biology, classification, and phenotype in cardiovascular disease: JACC macrophage in CVD series (part 1). J Am Coll Cardiol. 2018;72:2166–80.
Werner RA, Thackeray JT, Diekmann J, Weiberg D, Bauersachs J, Bengel FM. The changing face of nuclear cardiology: guiding cardiovascular care toward molecular medicine. J Nucl Med. 2020;61:951–61.
Paulos CM, Turk MJ, Breur GJ, Low PS. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv Drug Deliv Rev. 2004;56:1205–17.
Silvola JMU, Li X, Virta J, Marjamäki P, Liljenbäck H, Hytönen JP, et al. Aluminum fluoride-18 labeled folate enables in vivo detection of atherosclerotic plaque inflammation by positron emission tomography. Sci Rep. 2018;8:9720.
Ayala-López W, Xia W, Varghese B, Low PS. Imaging of atherosclerosis in apoliprotein e knockout mice: targeting of a folate-conjugated radiopharmaceutical to activated macrophages. J Nucl Med. 2010;51:768–74.
Jahandideh A, Uotila S, Stahle M, Virta J, Li XG, Kyto V, et al. Folate receptor β-targeted PET imaging of macrophages in autoimmune myocarditis. J Nucl Med. 2020;61:1643–9.
Guo Z, You L, Shi C, Song M, Gao M, Xu D, et al. Development of a new FR-targeting agent 99mTc-HYNFA with improved imaging contrast and comparison of multimerization and/or PEGylation strategies for radio-folate modification. Mol Pharm. 2017;14:3780–8.
Guo Z, Zhang P, Song M, Wu X, Liu C, Zhao Z, et al. Synthesis and preliminary evaluation of novel 99mTc-labeled folate derivative via click reaction for SPECT imaging. Appl Radiat Isotopes. 2014;91:24–30.
Guo Z, Gao M, Song M, Shi C, Zhang P, Xu D, et al. Synthesis and evaluation of 99mTc-labeled dimeric folic acid for FR-targeting. Molecules. 2016;21:817–29.
Guo Z, Yang L, Chen M, Wen X, Liu H, Li J, et al. Molecular imaging of advanced atherosclerotic plaques with folate receptor-targeted 2D nanoprobes. Nano Res. 2019;13:173–82.
Lau J, Jacobson O, Niu G, Lin KS, Bѐnard F, Chen X. Bench to bedside: albumin binders for improved cancer radioligand therapies. Bioconjug Chem. 2019;30:487–502.
Siwowska K, Haller S, Bortoli F, Benešová M, Groehn V, Bernhardt P, et al. Preclinical comparison of albumin-binding radiofolates: impact of linker entities on the in vitro and in vivo properties. Mol Pharm. 2017;14:523–32.
Deberle LM, Benešová M, Umbricht CA, Borgna F, Büchler M, Zhernosekov K, et al. Development of a new class of PSMA radioligands comprising ibuprofen as an albumin-binding entity. Theranostics. 2020;10:1678–93.
Müller C, Struthers H, Winiger C, Zhernosekov K, Schibli R. DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice. J Nucl Med. 2013;54:124–31.
Choy CJ, Ling X, Geruntho JJ, Beyer SK, Latoche JD, Langton-Webster B, et al. 177Lu-labeled phosphoramidate-based PSMA inhibitors: the effect of an albumin binder on biodistribution and therapeutic efficacy in prostate tumor-bearing mice. Theranostics. 2017;7:1928–39.
Wen X, Shi C, Xu D, Zhang P, Li Z, Li J, et al. Radioiodinated portable albumin binder as a versatile agent for in vivo imaging with single-photon emission computed tomography. Mol Pharm. 2019;16:816–24.
Rios FJ, Koga MM, Pecenin M, Ferracini M, Gidlund M, Jancar S. Oxidized LDL induces alternative macrophage phenotype through activation of CD36 and PAFR. Mediators of Inflamm. 2013;2013:198193.
Hu Y, Wu S, Zhao J, Ma X, Lu J, Xiu J, et al. VNN1 promotes atherosclerosis progression in apoE-/- mice fed a high-fat/high-cholesterol diet. J Lipid Res. 2016;57:1398–411.
Kelly JM, Amor-Coarasa A, Nikolopoulou A, Wüstemann T, Barelli P, Kim D, et al. Dual-target binding ligands with modulated pharmacokinetics for endoradiotherapy of prostate cancer. J Nucl Med. 2017;58:1442–9.
Dumelin CE, Trüssel S, Buller F, Trachsel E, Bootz F, Zhang Y, et al. A portable albumin binder from a DNA-encoded chemical library. Angew Chem Int Ed. 2008;47:3196–201.
Pais R, Redheuil A, Cluzel P, Ratziu V, Giral P. Relationship among fatty liver, specific and multiple-site atherosclerosis, and 10-year framingham score. Hepatol. 2019;69:1453–63.
Höltke C, Grewer M, Stölting M, Geyer C, Wildgruber M, Helfen A. Exploring the influence of different albumin binders on molecular imaging probe distribution. Mol Pharm. 2021. https://doi.org/10.1021/acs.molpharmaceut.1c00064.
Funding
This study was financially supported by the National Natural Science Foundation of China (81901805, 21976150), Major Research Plan of the National Natural Science Foundation of China (91959122), and Joint Fund of the National Natural Science Foundation of China—China National Nuclear Corporation for Nuclear Technology Innovation (U1967222).
Author information
Authors and Affiliations
Contributions
Zhide Guo and Xianzhong Zhang were responsible for the conception and design of the study, the drafting of the manuscript, and the final approval of the version to be published. Xuejun Wen was responsible for the acquisition, analysis and interpretation of the data, and the drafting of the manuscript. Rongqiang Zhuang and Haibo Zhu contributed to critical revision for important intellectual content and final approval of the version to be published. Jinxiong Huang and Yesen Li contributed to the critical revision of the manuscript for important intellectual content and material support. Xiaoru Lin assisted in the synthesis of probes. Changrong Shi, Liu Yang, and Xinying Zeng directed the histopathological characterization of animal models.
Corresponding authors
Ethics declarations
Ethics approval
Animal experiments were approved by the animal care committee of Xiamen University (ID XMULAC20190157) and carried out in compliance with the national laws related to the conduct of animal experimentation.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wen, X., Shi, C., Yang, L. et al. A radioiodinated FR-β-targeted tracer with improved pharmacokinetics through modification with an albumin binder for imaging of macrophages in AS and NAFL. Eur J Nucl Med Mol Imaging 49, 503–516 (2022). https://doi.org/10.1007/s00259-021-05447-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05447-4