Abstract
Purpose
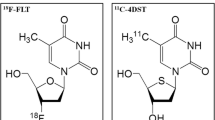
Positron emission tomography (PET) with 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT) provides a noninvasive assessment of tumour proliferation in vivo and could be a valuable imaging modality for assessing malignancy in meningiomas. We investigated a range of static and dynamic [18F]FLT metrics by correlating the findings with cellular biomarkers of proliferation and angiogenesis.
Methods
Seventeen prospectively recruited adult patients with intracranial meningiomas underwent a 60-min dynamic [18F]FLT PET following surgery. Maximum and mean standardized uptake values (SUVmax, SUVmean) with and without normalization to healthy brain tissue and blood radioactivity obtained from 40 to 60 min summed dynamic images (PET40–60) and ~ 60-min blood samples were calculated. Kinetic modelling using a two-tissue reversible compartmental model with a fractioned blood volume (VB) was performed to determine the total distribution volume (VT). Expressions of proliferation and angiogenesis with key parameters including Ki-67 index, phosphohistone-H3 (phh3), MKI67, thymidine kinase 1 (TK1), proliferating cell nuclear antigen (PCNA), Kirsten RAt Sarcoma viral oncogene homolog (KRAS), TIMP metallopeptidase inhibitor 3 (TIMP3), and vascular endothelial growth factor A (VEGFA) were determined by immunohistochemistry and/or quantitative polymerase chain reaction.
Results
Immunohistochemistry revealed 13 World Health Organization (WHO) grade I and four WHO grade II meningiomas. SUVmax and SUVmean normalized to blood radioactivity from PET40–60 and blood sampling, and VT were able to significantly differentiate between WHO grades with the best results for maximum and mean tumour-to-whole-blood ratios (sensitivity 100%, specificity 94–95%, accuracy 99%; P = 0.003). Static [18F]FLT metrics were significantly correlated with proliferative biomarkers, especially Ki-67 index, phh3, and TK1, while no correlations were found with VEGFA or VB. Using Ki-67 index with a threshold > 4%, the majority of [18F]FLT metrics showed a high ability to identify aggressive meningiomas with SUVmean demonstrating the best performance (sensitivity 80%, specificity 81%, accuracy 80%; P = 0.024).
Conclusion
[18F]FLT PET could be a useful imaging modality for assessing cellular proliferation in meningiomas.




Similar content being viewed by others
Abbreviations
- BBB:
-
Blood-brain barrier
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- CT :
-
Cycle threshold
- EANO:
-
European Association of Neuro-Oncology
- EGFR:
-
Epidermal growth factor receptor
- [18F]FDG:
-
2-[18F]fluoro-2-deoxy-D-glucose
- [18F]FET:
-
O-(2-[18F]fluoroethyl)-L-tyrosine
- [18F]FLT:
-
3′-deoxy-3′-[18F]fluorothymidine
- IDIF:
-
Image-derived input function
- GOI:
-
Genes of interest
- IQR:
-
Interquartile range
- KRAS:
-
Kirsten RAt Sarcoma viral oncogene homologue
- [11C]MET:
-
[Methyl-11C]-L-methionine
- MGAT5:
-
N-Acetylglucosaminyltransferase V
- MMP2/9:
-
Matrix metallopeptidases 2 and 9
- MRI:
-
Magnetic resonance imaging
- PCNA:
-
Proliferating cell nuclear antigen
- PET:
-
Positron emission tomography
- PF:
-
Parent fraction
- phh3:
-
Phosphohistone-H3
- qPCR:
-
Quantitative polymerase chain reaction
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- SSTR2:
-
Somatostatin receptor subtype 2
- SUV:
-
Standardized uptake value
- TAC:
-
Time-activity curve
- TBR:
-
Tumour-to-blood ratio
- TGR:
-
Tumour-to-grey ratio
- TIMP3/4:
-
TIMP metallopeptidase inhibitors 3 and 4
- TK1:
-
Thymidine kinase 1
- TPFR:
-
Tumour-to-parent fraction ratio
- TPR:
-
Tumour-to-plasma ratio
- TWBR:
-
Tumour-to-whole-blood ratio
- TWR:
-
Tumour-to-white matter ratio
- V B :
-
Blood volume fraction
- VEGFA:
-
Vascular endothelial growth factor A
- VOI:
-
Volume of interest
- WHO:
-
World Health Organization
References
Backer-Grøndahl T, Moen BH, Torp SH. The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol. 2012;5:231–42.
Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20.
Pearson BE, Markert JM, Fisher WS, et al. Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008;24:E3.
Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17:e383–91.
Rogers L, Zhang P, Vogelbaum MA, et al. Intermediate-risk meningioma: initial outcomes from NRG Oncology RTOG 059. J Neurosurg. 2018;129:35–47.
Chalkidou A, Landau DB, Odell EW, et al. Correlation between Ki-67 immunohistochemistry and 18F-Fluorothymidine uptake in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2012;48:3499–513.
Rasey JS, Grierson JR, Wiens LW, et al. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med. 2002;43:1210–7.
Barthel H, Perumal M, Latigo J, et al. The uptake of 3′-deoxy-3′-[18F]fluorothymidine into L5178Y tumours in vivo is dependent on thymidine kinase 1 protein levels. Eur J Nucl Med Mol Imaging. 2005;32:257–63.
Wagner M, Seitz U, Buck A, et al. 3′-[F-18]fluoro-3′-deoxythymidine ([F-18]-FLT) as positron emission tomography tracer for imaging proliferation in a murine B-cell lymphoma model and in the human disease. Cancer Res. 2008;63:2681–7.
Wang H, Zhang J, Tian J, et al. Using dual-tracer PET to predict the biologic behavior of human colorectal cancer. J Nucl Med. 2009;50:1857–64.
Brockenbrough JS, Souquet T, Morihara JK, et al. Tumor 3′-deoxy-3′-(18)F-fluorothymidine ((18)F-FLT) uptake by PET correlates with thymidine kinase 1 expression: static and kinetic analysis of (18)F-FLT PET studies in lung tumors. J Nucl Med. 2011;52:1181–8.
McKinley ET, Ayers GD, Smith A, et al. Limits of [18F]-FLT PET as a biomarker of proliferation in oncology. PLoS One. 2013;8:e58938.
Zhang CC, Yan Z, Li W, et al. [18F]FLT-PET imaging does not always “light up” proliferating tumor cells. Clin Cancer Res. 2003;63:2681–7.
Jacobs AH, Thomas A, Kracht LW, et al. 18F-fluro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med. 2005;46:1948–58.
Muzi M, Spence AM, O’Sullivan F, et al. Kinetic analysis of 3′-deoxy-3’-18F-fluorothymidine in patients with gliomas. J Nucl Med. 2006;47:1612–21.
Schiepers C, Chen W, Dahlbom M, et al. (18)F-fluorothymidine kinetics of malignant brain tumors. Eur J Nucl Med Mol Imaging. 2007;34:1003–11.
Blocher A, Kuntzsch M, Wei R, Machulla HJ. Synthesis and labeling of 5-O-(4,4-dimethoxytrityl)-2,3′-anhydrothymidine for [18F]FLT preparation. J Radioanal Nucl Chem. 2002;251:55–8.
Shields AF, Briston DA, Chandupatla S, et al. A simplified analysis of [18F]3′-deoxy-3′-fluorothymidine metabolism and retention. Eur J Nucl Med Mol Imaging. 2005;32:1269–75.
Ladefoged CN, Andersen FL, Kjær A, et al. RESOLUTE PET/MRI attenuation correction for O-(2-18F-fluoroethyl)-L-tyrosine (FET) in brain tumor patients with metal implants. Front Neurosci. 2017;11:453.
Khalighi MM, Deller TW, Fan AP, et al. Image-derived input function estimation on a TOF-enabled PET/MR for cerebral blood flow mapping. J Cereb Blood Flow Metab. 2018;38:126–35.
Domingues P, González-Tablas M, Otero Á, et al. Genetic/molecular alterations of meningiomas and the signalling pathways targeted. Oncotarget. 2015;6:10671–88.
Gupta S, Linda W, Dunn IF. Medical management of meningioma in the era of precision medicine. Neurosurg Focus. 2018;44:E3.
Carstens C, Messe E, Zang KD, Blin N. Human KRAS oncogene expression in meningioma. Cancer Lett. 1988;43:37–41.
Barski D, Wolter M, Reifenberger G, Riemenschneider MJ. Hypermethylation and transcriptional downregulation of the TIMP3 gene is associated with allelic loss on 22q12. 3 and malignancy in meningiomas. Brain Pathol. 2010;20:623–31.
Nagae M, Kizuka Y, Mihara E, et al. Structure and mechanism of cancer-associated N-acetylglucosaminyltransferase-V. Nat Commun. 2018;9:3380.
Okada M, Miyake K, Matsumoto Y, et al. Matrix metalloproteinase-2 and matrix metalloproteinase-9 expressions correlate with the recurrence of intracranial meningiomas. J Neuro-Oncol. 2004;66:29–37.
Okuducu AF, Zils U, Michaelis SA, et al. Increased expression of avian erythroblastosis virus E26 oncogene homolog 1 in World Health Organization grade 1 meningiomas is associated with an elevated risk of recurrence and is correlated with the expression of its target genes matrix metalloproteinase-2 and MMP-9. Cancer. 2006;107:1365–72.
Yamamoto Y, Ono Y, Aga F, et al. Correlation of 18F-FLT uptake with tumor grade and Ki-67 immunohistochemistry in patients with newly diagnosed and recurrent gliomas. J Nucl Med. 2012;53:1911–5.
Chen W, Cloughesy T, Kamdar N, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005;46:945–52.
Galldiks N, Lohmann P, Albert NL, et al. Current status of PET imaging in neuro-oncology. Neuro-Oncology. 2019. https://doi.org/10.1093/noajnl/vdz010.
Nowosielski M, Difranco MD, Putzer D, et al. An intra-individual comparison of MRI, [18F]-FET and [18F]-FLT PET in patients with high-grade gliomas. PLoS One. 2014;9:e95830.
Tripathi M, Sharma R, D’Souza M, et al. Comparative evaluation of F-18 FDOPA, F-18 FDG, and F-18 FLT-PET/CT for metabolic imaging of low grade gliomas. Clin Nucl Med. 2009;34:878–83.
Shinomiya A, Kawai N, Okada M, et al. Evaluation of 3′-deoxy-3′-[18F]-fluorothymidine (18F-FLT) kinetics correlated with thymidine kinase-1 expression and cell proliferation in newly diagnosed gliomas. Eur J Nucl Med Mol Imaging. 2013;40:175–85.
Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–9.
Nikaki A, Prassopoulos V, Efthimiadou R, et al. FLT PET/CT in a case of demyelinating disease. Clin Nucl Med. 2016;41:e342–5.
Holter JL, Thorp K, Smith ML, et al. [18F]Fluorothymidine PET imaging in the diagnosis of leptomeningeal involvement with diffuse large B-cell lymphoma. Cancer Imaging. 2011;11:140–3.
Anton-Rodriguez JM, Lewis D, Djoukhadar I, et al. [18F]flurothymidine and [18F]flurodeoxyglucose PET imaging demonstrates uptake and differentiates growth in neurofibromatosis 2 related vestibular schwannoma. Otol Neurootol. 2019;40:826–35.
Huang RY, Bi WL, Weller M, et al. Proposed response assessment and endpoints for meningioma clinical trials: report from the response assessment in neuro-oncology working group. Neuro-Oncol. 2019;21:26–36.
Nguyen NC, Yee MK, Tuchayi AM, et al. Targeted therapy and immunotherapy response assessment with F-18-Flourothymidine positron-emission-tomography/magnetic resonance imaging in melanoma brain metastasis: a pilot study. Front Oncol. 2018;8:18.
Lee JW, Kang KW, Park SH, et al. 18F-FDG PET in the assessment of tumor grade and prediction of tumor recurrence in intracranial meningioma. Eur J Nucl Med Mol Imaging. 2009;36:1574–82.
Mitamura K, Yamamoto Y, Norikane T, et al. Correlation of 18F-FDG and 11C-methionine uptake in PET/CT with Ki-67 immunohistochemistry in newly diagnosed intracranial meningiomas. Ann Nucl Med. 2018;32:627–33.
Arita H, Kinoshita M, Okita Y, et al. Clinical characteristics of meningiomas assessed by 11C-methionine and 18F-fluorodeoxyglucose positron-emission tomography. J Neuro-Oncol. 2012;107:379–86.
Ogawa T, Inugami A, Hatazawa J, et al. Clinical positron emission tomography for brain tumors: comparison of fludeoxyglucose F18 and L-methyl-11C-methionine. AJNR Am J Neuroradiol. 1996;17:345–53.
Cornelius JF, Stoffels G, Filß C, et al. Uptake and tracer kinetics of O-(2-(18)F-fluoroethyl)-L-tyrosine in meningiomas: preliminary results. Eur J Nucl Med Mol Imaging. 2015;42:459–67.
Muzi M, Mankoff DA, Grierson JR, et al. Kinetic modeling of 3′-deoxy-3′-fluorothymidine in somatic tumors: mathematical studies. J Nucl Med. 2005;46:371–80.
Acknowledgements
The authors would like to thank the technical staff Nadia Azizi, Marianne Federspiel, Jakup Martin Poulsen, and Karin Stahr and chemists at Clinic of Clinical Physiology, Nuclear Medicine & PET, for help with PET scanning and patient care. The authors would also like to thank the nuclear medicine specialist, Oriol Puig, for helping with kinetic modelling images for the study. Finally, a special thanks to the statistician, Lasse Anderberg, for helping with ROC analyses.
Funding
The work was supported by a grant from the Danish Cancer Society (R146-A9508–16-S2). The Siemens mMR hybrid PET/MR system at Copenhagen University Hospital Rigshospitalet was donated by the John and Birthe Meyer Foundation.
Author information
Authors and Affiliations
Contributions
Asma Bashir, Ian Law, Morten Ziebell, and Kåre Fugleholm conceived the study design. Asma Bashir was responsible for patient recruitment. Asma Bashir and Ian Law contributed to the acquisition, analysis, and interpretation of the PET/MRI data. Asma Bashir, Mark B. Vestergaard, and Lisbeth Marner conducted full kinetic modelling using an image-derived input function. Tina Binderup performed quantitative polymerase chain reaction. Helle Broholm performed the neuropathological examination. Asma Bashir conducted the statistical analysis with the guidance from senior statisticians, Thomas Sheike (Professor) and Lasse Anderberg (MSc). Asma Bashir drafted the first and subsequent manuscripts. Asma Bashir, Mark B. Vestergaard, Helle Broholm, Tina Binderup, Lisbeth Marner, Morten Ziebell, Kåre Fugleholm, Andreas Kjær, and Ian Law contributed to the data interpretation, the increase in the intellectual content, and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Scientific Health Ethics Committee of the Capital region, Copenhagen, Denmark (reference number: H-15006091) and was based on the Declaration of Helsinki II (2013) and the principles of “good clinical practices”.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology—Brain.
Electronic supplementary material
ESM 1
(DOC 317 kb)
Rights and permissions
About this article
Cite this article
Bashir, A., Binderup, T., Vestergaard, M.B. et al. In vivo imaging of cell proliferation in meningioma using 3′-deoxy-3′-[18F]fluorothymidine PET/MRI. Eur J Nucl Med Mol Imaging 47, 1496–1509 (2020). https://doi.org/10.1007/s00259-020-04704-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04704-2