Abstract
Purpose
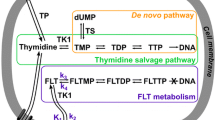
The aim of this study was to investigate the role of thymidine kinase 1 (TK1) protein in 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT) positron emission tomography (PET) studies.
Methods
We investigated the in vivo kinetics of [18F]FLT in TK1+/− and TK1−/− L5178Y mouse lymphoma tumours that express different levels of TK1 protein.
Results
[18F]FLT-derived radioactivity, measured by a dedicated small animal PET scanner, increased within the tumours over 60 min. The area under the normalised tumour time–activity curve were significantly higher for the TK1+/− compared with the −/− variant (0.89±0.02 vs 0.79±0.03 MBq ml−1 min, P=0.043; n=5 for each tumour type). Ex vivo gamma counting of tissues excised at 60 min p.i. (n=8) also revealed significantly higher tumour [18F]FLT uptake for the TK1+/− variant (6.2±0.6 vs 4.6±0.4%ID g−1, P=0.018). The observed differences between the cell lines with respect to [18F]FLT uptake were in keeping with a 48% higher TK1 protein in the TK1+/− tumours versus the −/− variant (P=0.043). On average, there were no differences in ATP levels between the two tumour variants (P=1.00). A positive correlation between [18F]FLT accumulation and TK1 protein levels (r=0.68, P=0.046) was seen. Normalisation of the data for ATP content further improved the correlation (r=0.86, P=0.003).
Conclusion
This study shows that in vivo [18F]FLT kinetics depend on TK1 protein expression. ATP may be important in realising this effect. Thus, [18F]FLT-PET has the potential to yield specific information on tumour proliferation in diagnostic imaging and therapy monitoring.




Similar content being viewed by others
References
Owa T, Yoshino H, Yoshimatsu K, Nagasu T. Cell cycle regulation in the G1 phase: a promising target for the development of new chemotherapeutic anticancer agents. Curr Med Chem 2001;8(12):1487–503.
Swanton C. Cell-cycle targeted therapies. Lancet Oncol 2004;5:27–36.
Workman P. How much gets there and what does it do? the need for better pharmacokinetic and pharmacodynamic endpoints in contemporary drug discovery and development. Curr Pharm Des 2003;9:891–902.
Hutchinson OC, Collingridge DR, Barthel H, Price PM, Aboagye EO. Pharmacokinetics of radiolabelled anticancer drugs for positron emission tomography. Curr Pharm Des 2003;9:917–29.
Hutchinson OC, Collingridge DR, Barthel H, Price PM, Aboagye EO. Pharmacodynamics of radiolabelled anticancer drugs for positron emission tomography. Curr Pharm Des 2003;9:931–44.
Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, et al. Imaging proliferation in vivo with [18F]FLT and positron emission tomography. Nat Med 1998;4:1334–6.
Toyohara J, Waki A, Takamatsu S, Yonekura Y, Magata Y, Fujibayashi Y. Basis of FLT as a cell proliferation marker: comparative uptake studies with [3H]thymidine and [3H]arabinothymidine, and cell-analysis in 22 asynchronously growing tumor cell lines. Nucl Med Biol 2002;29:281–7.
Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med 2002;43:1210–17.
Schwartz JL, Tamura Y, Jordan R, Grierson JR, Krohn KA. Monitoring tumor cell proliferation by targeting DNA synthetic processes with thymidine and thymidine analogs. J Nucl Med 2003;44:2027–32.
Barthel H, Cleij MC, Collingridge DR, Hutchinson OC, Osman S, He Q, et al. 3′-Deoxy-3′-[18F]fluorothymidine as a new marker for monitoring tumor response to anti-proliferative therapy in vivo with positron emission tomography. Cancer Res 2003;63:3791–98.
Buck AK, Schirrmeister H, Hetzel M, Von Der Heide M, Halter G, Glatting G, et al. 3-Deoxy-3-[18F]fluorothymidine-positron emission tomography for noninvasive assessment of proliferation in pulmonary nodules. Cancer Res 2002;62:3331–4.
Vesselle H, Grierson J, Muzi M, Pugsley JM, Schmidt RA, Rabinowitz P, et al. In vivo evaluation of 3′doxy-3′-[18F]fluorothymidine ([18F]FLT) as a proliferation imaging tracer in humans: correlation of [18F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res 2002;8:3315–23.
Buck AK, Halter G, Schirrmeister H, Kotzerke J, Wurziger I, Glatting G, et al. Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J Nucl Med 2003;44:1426–31.
Dittmann H, Dohmen BM, Paulsen F, Eichhorn K, Eschmann SM, Horger M, et al. [18F]FLT PET for diagnosis and staging of throacic tumours. Eur J Nucl Med Mol Imaging 2003;30:1407–12.
Francis DL, Freeman A, Visvikis D, Costa DC, Luthra SK, Novelli M, et al. In vivo imaging of cellular proliferation in colorectal cancer using positron emission tomography. Gut 2003;52:1602–6.
Cobben DC, Elsinga PH, Suurmeijer AJ, Vaalburg W, Maase B, Jager PL, Hoekstra HJ. Detection and grading of soft tissue sarcomas of the extremities with 18F-3′-fluoro-3′-deoxy-l-thymidine. Clin Cancer Res 2004;10:1685–90.
Ellims PH, Van der Weyden MB, Medley G. Thymidine kinase isoenzymes in human malignant lymphoma. Cancer Res 1981;41:691–5.
Sherley JL, Kelly TJ. Regulation of human thymidine kinase during the cell cycle. J Biol Chem 1988;263:8350–8.
Chang ZF, Huang DY, Hsue NC. Differential phosphorylation of human thymidine kinase in proliferating and M phase-arrested human cells. J Biol Chem 1994;269:21249–54.
Munch-Petersen B, Cloos L, Jensen HK, Tyrsted G. Human thymidine kinase. I. Regulation in normal and malignant cells. Adv Enzyme Regul 1995;35:69–89.
Olofsson S, Eriksson S, Karlsson A, Oberg B. The HIV replication inhibitor 3′-fluoro-3′-deoxythymidine blocks sialylation of N-linked oligosaccharides. Antiviral Res 1992;19:71–80.
Cleij MC, Steel CJ, Brady F, Ell PJ, Pike VW, Luthra SK. An improved synthesis of 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT) and the fate of the precursor 2,3′-anhydro-5(-O-(4,4′-dimethoxytrityl)-thymidine. J Label Comput Radiopharm 2001;44(Suppl 1):871–3.
Stephens TC, Smith MN, Waterman SE, McCloskey ML, Jackman A, Boyle FT. Use of murine L5178Y lymphoma thymidine kinase mutants for in vitro and in vivo antitumour efficacy evaluation of novel thymidylate synthase inhibitors. Adv Exp Med Biol 1993;338:589–92.
Aherne GW, Hardcastle A, Ward E, Dobinson D, Crompton T, Valenti M, et al. Pharmacokinetic/pharmacodynamic study of ZD9331, a nonpolyglutamatable inhibitor of thymidylate synthase, in a murine model following two curative administration schedules. Clin Cancer Res 2001;7:2923–30.
Honma M, Hayashi H, Shimada N, Tanaka N, Wakuri S, Awogi T, et al. Evaluation of the mouse lymphoma tk assay (microwell method) as an alternative to the in vitro chromosomal aberration test. Mutagenesis 1999;14:5–22.
Chen T, Harrington-Brock K, Moore MM. Mutant frequencies and loss of heterozygosity induced by N-ethyl-N-nitrosourea in the thymidine kinase gene of L5178Y/TK+/−-3.7.2C mouse lymphoma cells. Mutagenesis 2002;17:105–9.
Combes RD, Stopper H, Caspary WJ. The use of lymphoma cells to assess the mutagenic, clastogenic and aneugenic properties of chemicals. Mutagenesis 1995;10:403–8.
Barthel H, Wilson H, Collingridge DR, Brown G, Osman S, Luthra SK, et al. In vivo evaluation of [18F]fluoroetanidazole as a new marker for imaging tumour hypoxia with positron emission tomography. Br J Cancer 2004;90:2232–42.
Tommasi S, Pfeifer GP. Constitutive protection of E2F recognition sequences in the human thymidine kinase promotor during cell cycle progression. J Biol Chem 1997;272:30483–90.
Liechty MC, Rauchfuss HS, Lugo MH, Hozier JC. Sequence analysis of tka(−)-1 and tkb(+)-1 alleles in L5178Y tk+/− mouse-lymphoma cells and spontaneous tk−/− mutants. Mutat Res 1993;286:299–307.
Clive D, Glover P, Krehl R, Poorman-Allen P. Mutagenicity of 2-amino-N6-hydroxyadenine (AHA) at three loci in L5178Y/tk+/− mouse lymphoma cells: molecular and preliminary cytogenetic characterizations of AHA-induced tk−/− mutants. Mutat Res 1991;253:73–82.
Oberly TJ, Bewsey BJ, Probst GS. Thymidine kinase activity and trifluorothymidine resistance of spontaneous and mutagen-induced L5178Y cells in RPMI 1640 medium. Mutat Res 1986;161:165–71.
Kristensen T, Jensen HK, Munch-Petersen B. Overexpression of human thymidine kinase mRNA without corresponding enzymatic activity in patients with chronic lymphatic leukemia. Leuk Res 1994;18:861–6.
Kauffman MG, Kelly TJ. Cell cycle regulation of thymidine kinase: residues near the carboxyl terminus are essential for the specific degradation of the enzyme at mitosis. Mol Cell Biol 1991;11:2538–46.
United Kingdom Coordinating Committee on Cancer Research (UKCCCR) Guidelines for the welfare of animals in experimental neoplasia (second edition). Br J Cancer 1998;77:1–10.
Acknowledgements
The study was supported by Cancer Research UK grants C2536/A3554 and C153/A1802. H.B. was supported by a grant awarded by the Leopoldina Society (Halle, Germany) and administered through the German Education and Research Ministry (BMBF-LPD 9901/8-22). The authors also wish to thank Marcel C. Cleij for synthesising the [18F]FLT, Kawai Yau for her assistance in the biodistribution studies and Lynn Maslen for her editorial support in the completion of the manuscript. We are grateful to David Gibbs and Ann Jackman for providing the L5178Y cell lines. The animal experiments were performed by licensed investigators in accordance with the national guidelines [35].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barthel, H., Perumal, M., Latigo, J. et al. The uptake of 3′-deoxy-3′-[18F]fluorothymidine into L5178Y tumours in vivo is dependent on thymidine kinase 1 protein levels. Eur J Nucl Med Mol Imaging 32, 257–263 (2005). https://doi.org/10.1007/s00259-004-1611-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1611-0