Abstract
Purpose
To assess the clinical utility of 18F-Fluorodeoxyglucose positron emission tomography (FDG-PET) for detection of early signs of neurodegeneration in conditions of increased risk for Alzheimer’s disease (AD) as defined by: subjective cognitive decline (SCD), evidence of cerebral amyloid-pathology, apolipoprotein E (APOE) ε4-positive genotype, or autosomal dominant forms of AD (ADAD) in asymptomatic stages.
Methods
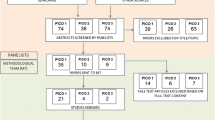
A comprehensive literature search was conducted using the PICO model to extract evidence from relevant studies. An expert panel then voted using the Delphi method on three different diagnostic scenarios.
Results
The level of empirical study evidence for the use of FDG-PET to detect meaningful early signs of neurodegeneration was considered to be poor for ADAD and lacking for SCD and asymptomatic persons at risk, based on APOE ε4-positive genotype or cerebral amyloid pathology. Consequently, and consistent with current diagnostic criteria, panelists decided not to recommend routine clinical use of FDG-PET in these situations and to currently mainly reserve it for research purposes.
Conclusion
Currently, there is limited evidence on which to base recommendations regarding the clinical routine use of FDG-PET to detect diagnostically meaningful early signs of neurodegeneration in asymptomatic subjects with ADAD, with APOE ε4-positive genotype, or with cerebral amyloid pathology, and in subjects with SCD. Future prospective studies are warranted and in part already ongoing, aiming to assess the added value of FDG-PET in this context beyond research applications.

Similar content being viewed by others
References
Nobili F, Arbizu J, Bouwman F, Drzezga A, Filippi M, Nestor P, et al. EAN–EANM recommendations for the use of brain 18F-Fluorodeoxyglucose positron emission tomography (FDG-PET) in neurodegenerative cognitive impairment and dementia: Delphi consensus. Eur J Neurol. n.d.
Rabin LA, Smart CM, Crane PK, Amariglio RE, Berman LM, Boada M, et al. Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. J Alzheimers Dis. 2015;48(Suppl 1):S63–86. https://doi.org/10.3233/JAD-150154.
Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10:844–52. https://doi.org/10.1016/j.jalz.2014.01.001.
Wolfsgruber S, Kleineidam L, Wagner M, Mösch E, Bickel H, Lϋhmann D, et al. Differential risk of incident Alzheimer’s disease dementia in stable versus unstable patterns of subjective cognitive decline. J Alzheimers Dis. 2016;54:1135–46. https://doi.org/10.3233/JAD-160407.
Villemagne VL, Pike KE, Chételat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–92. https://doi.org/10.1002/ana.22248.
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. https://doi.org/10.1016/j.jalz.2011.03.003.
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3.
Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther. 2011;3:1. https://doi.org/10.1186/alzrt59.
Boccardi M, Festari C, Altomare D, Gandolfo F, Orini S, Nobili F, et al. Assessing accuracy diagnostic FDG-PET studies to define clinical use for dementia diagnosis. EJNMMI in this issue.
Leone MA, Brainin M, Boon P, Pugliatti M, Keindl M, Bassetti CL. Guidance for the preparation of neurological management guidelines by EFNS scientific task forces - revised recommendations 2012. Eur J Neurol. 2013;20:410–9. https://doi.org/10.1111/ene.12043.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. https://doi.org/10.1016/j.jclinepi.2009.06.005.
Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. https://doi.org/10.1016/j.jalz.2016.02.002.
Schroeter ML, Vogt B, Frisch S, Becker G, Seese A, Barthel H, et al. Dissociating behavioral disorders in early dementia—an FDG-PET study. Psychiatry Res. 2011;194:235–44. https://doi.org/10.1016/j.pscychresns.2011.06.009.
Picco A, Polidori MC, Ferrara M, Cecchetti R, Arnaldi D, Baglioni M, et al. Plasma antioxidants and brain glucose metabolism in elderly subjects with cognitive complaints. Eur J Nucl Med Mol Imaging. 2014;41:764–75. https://doi.org/10.1007/s00259-013-2638-x.
Auning E, Selnes P, Grambaite R, Šaltyte Benth J, Haram A, Løvli Stav A, et al. Neurobiological correlates of depressive symptoms in people with subjective and mild cognitive impairment. Acta Psychiatr Scand. 2015;131:139–47. https://doi.org/10.1111/acps.12352.
Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008;63:609–18. https://doi.org/10.1016/j.biopsych.2007.05.030.
Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kölsch H, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79:1332–9. https://doi.org/10.1212/WNL.0b013e31826c1a8d.
Van Der Gucht A, Verger A, Yagdigul Y, Poussier S, Joly L, Watfa G, et al. Complementarity of visual and voxel-based FDG-PET analysis to detect MCI-like hypometabolic pattern in elderly patients with hypertension and isolated memory complaints. Acta Radiol. 2015;56:980–9. https://doi.org/10.1177/0284185114542366.
Brugnolo A, Morbelli S, Arnaldi D, De Carli F, Accardo J, Bossert I, et al. Metabolic correlates of Rey auditory verbal learning test in elderly subjects with memory complaints. J Alzheimers Dis. 2014;39:103–13. https://doi.org/10.3233/JAD-121684.
Dowling NM, Johnson SC, Gleason CE, Jagust WJ, Alzheimer’s Disease Neuroimaging Initiative. The mediational effects of FDG hypometabolism on the association between cerebrospinal fluid biomarkers and neurocognitive function. NeuroImage. 2015;105:357–68. https://doi.org/10.1016/j.neuroimage.2014.10.050.
Ewers M, Insel PS, Stern Y, Weiner MW, Alzheimer’s Disease Neuroimaging Initiative (ADNI). Cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology. 2013;80:1194–201. https://doi.org/10.1212/WNL.0b013e31828970c2.
Ewers M, Brendel M, Rizk-Jackson A, Rominger A, Bartenstein P, Schuff N, et al. Reduced FDG-PET brain metabolism and executive function predict clinical progression in elderly healthy subjects. NeuroImage Clin. 2014;4:45–52. https://doi.org/10.1016/j.nicl.2013.10.018.
Petrie EC, Cross DJ, Galasko D, Schellenberg GD, Raskind MA, Peskind ER, et al. Preclinical evidence of Alzheimer changes: convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol. 2009;66:632–7. https://doi.org/10.1001/archneurol.2009.59.
Knopman DS, Jack CR, Wiste HJ, Lundt ES, Weigand SD, Vemuri P, et al. 18F-fluorodeoxyglucose positron emission tomography, aging, and apolipoprotein E genotype in cognitively normal persons. Neurobiol Aging. 2014;35:2096–106. https://doi.org/10.1016/j.neurobiolaging.2014.03.006.
Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:3334–9. https://doi.org/10.1073/pnas.061509598.
Chen K, Ayutyanont N, Langbaum JBS, Fleisher AS, Reschke C, Lee W, et al. Correlations between FDG PET glucose uptake-MRI gray matter volume scores and apolipoprotein E 3/4 gene dose in cognitively normal adults: a cross-validation study using voxel-based multi-modal partial least squares. NeuroImage. 2012;60:2316–22. https://doi.org/10.1016/j.neuroimage.2012.02.005.
Protas HD, Chen K, Langbaum JBS, Fleisher AS, Alexander GE, Lee W, et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 2013;70:320–5. https://doi.org/10.1001/2013.jamaneurol.286.
Langbaum JBS, Chen K, Caselli RJ, Lee W, Reschke C, Bandy D, et al. Hypometabolism in Alzheimer-affected brain regions in cognitively healthy Latino individuals carrying the apolipoprotein E epsilon4 allele. Arch Neurol. 2010;67:462–8. https://doi.org/10.1001/archneurol.2010.30.
Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101:284–9. https://doi.org/10.1073/pnas.2635903100.
Saint-Aubert L, Payoux P, Hannequin D, Barbeau EJ, Campion D, Delisle M-B, et al. MR, 18F-FDG, and 18F-AV45 PET correlate with AD PSEN1 original phenotype. Alzheimer Dis Assoc Disord. 2013;27:91–4. https://doi.org/10.1097/WAD.0b013e318251d87c.
Ting SKS, Benzinger T, Kepe V, Fagan A, Coppola G, Porter V, et al. A novel PSEN1 mutation (I238M) associated with early-onset Alzheimer’s disease in an African-American woman. J Alzheimers Dis. 2014;40:271–5. https://doi.org/10.3233/JAD-131844.
Uttner I, Kirchheiner J, Tumani H, Mottaghy FM, Lebedeva E, Ozer E, et al. A novel presenilin1 mutation (Q223R) associated with early onset Alzheimer’s disease, dysarthria and spastic paraparesis and decreased Abeta levels in CSF. Eur J Neurol. 2010;17:631–3. https://doi.org/10.1111/j.1468-1331.2009.02810.x.
Ringman JM, Gylys KH, Medina LD, Fox M, Kepe V, Flores DL, et al. Biochemical, neuropathological, and neuroimaging characteristics of early-onset Alzheimer’s disease due to a novel PSEN1 mutation. Neurosci Lett. 2011;487:287–92. https://doi.org/10.1016/j.neulet.2010.10.039.
Schöll M, Almkvist O, Axelman K, Stefanova E, Wall A, Westman E, et al. Glucose metabolism and PIB binding in carriers of a His163Tyr presenilin 1 mutation. Neurobiol Aging. 2011;32:1388–99. https://doi.org/10.1016/j.neurobiolaging.2009.08.016.
Nikisch G, Hertel A, Kiessling B, Wagner T, Krasz D, Hofmann E, et al. Three-year follow-up of a patient with early-onset Alzheimer’s disease with presenilin-2 N141I mutation — case report and review of the literature. Eur J Med Res. 2008;13:579–84.
Mosconi L, Sorbi S, Nacmias B, De Cristofaro MTR, Fayyaz M, Cellini E, et al. Brain metabolic differences between sporadic and familial Alzheimer’s disease. Neurology. 2003;61:1138–40.
Laforce R, Buteau JP, Paquet N, Verret L, Houde M, Bouchard RW. The value of PET in mild cognitive impairment, typical and atypical/unclear dementias: a retrospective memory clinic study. Am J Alzheimers Dis Other Dement. 2010;25:324–32. https://doi.org/10.1177/1533317510363468.
Schöll M, Almkvist O, Bogdanovic N, Wall A, Långström B, Viitanen M, et al. Time course of glucose metabolism in relation to cognitive performance and postmortem neuropathology in Met146Val PSEN1 mutation carriers. J Alzheimers Dis. 2011;24:495–506. https://doi.org/10.3233/JAD-2011-101563.
Mosconi L, Sorbi S, de Leon MJ, Li Y, Nacmias B, Myoung PS, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J Nucl Med. 2006;47:1778–86.
Schöll M, Carter SF, Westman E, Rodriguez-Vieitez E, Almkvist O, Thordardottir S, et al. Early astrocytosis in autosomal dominant Alzheimer’s disease measured in vivo by multi-tracer positron emission tomography. Sci Rep. 2015;5:16404. https://doi.org/10.1038/srep16404.
Yau W-YW, Tudorascu DL, McDade EM, Ikonomovic S, James JA, Minhas D, et al. Longitudinal assessment of neuroimaging and clinical markers in autosomal dominant Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2015;14:804–13. https://doi.org/10.1016/S1474-4422(15)00135-0.
Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. https://doi.org/10.1056/NEJMoa1202753.
Benzinger TLS, Blazey T, Jack CR, Koeppe RA, Su Y, Xiong C, et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci. 2013;110:E4502–9. https://doi.org/10.1073/pnas.1317918110.
Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A. 2005;102:8299–302. https://doi.org/10.1073/pnas.0500579102.
Vannini P, Hedden T, Huijbers W, Ward A, Johnson KA, Sperling RA. The ups and downs of the posteromedial cortex: age- and amyloid-related functional alterations of the encoding/retrieval Flip in cognitively normal older adults. Cereb Cortex. 2013;23:1317–28. https://doi.org/10.1093/cercor/bhs108.
Nordberg A, Carter SF, Rinne J, Drzezga A, Brooks DJ, Vandenberghe R, et al. A European multicentre PET study of fibrillar amyloid in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2013;40:104–14. https://doi.org/10.1007/s00259-012-2237-2.
Guze BH, Hoffman JM, Mazziotta JC, Baxter LR, Phelps ME. Positron emission tomography and familial Alzheimer’s disease: a pilot study. J Am Geriatr Soc. 1992;40:120–3.
Kennedy AM, Rossor MN, Frackowiak RS. Positron emission tomography in familial Alzheimer disease. Alzheimer Dis Assoc Disord. 1995;9:17–20.
Vannini P, Hanseeuw B, Munro CE, Amariglio RE, Marshall GA, Rentz DM, et al. Hippocampal hypometabolism in older adults with memory complaints and increased amyloid burden. Neurology. 2017;88:1759–67. https://doi.org/10.1212/WNL.0000000000003889.
Garibotto V, Herholz K, Boccardi M, Picco A, Varrone A, Nordberg A, et al. Clinical validity of brain fluorodeoxyglucose positron emission tomography as a biomarker for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol Aging. 2017;52:183–95. https://doi.org/10.1016/j.neurobiolaging.2016.03.033.
Acknowledgements
The procedure for assessing scientific evidence and defining consensual recommendations was funded by the European Association of Nuclear Medicine (EANM) and by the European Academy of Neurology (EAN). We thank the Guidelines working group of EAN, particularly Simona Arcuti and Maurizio Leone, for methodological advice.
Funding
This project was partially funded by the European Association of Nuclear Medicine (EANM) and the European Academy of Neurology (EAN).
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Conflict of interest
Flavio Nobili: received personal fees and non-financial support from GE Healthcare, non-financial support from Eli-Lilly, and grants from Chiesi Farmaceutici.
Cristina Festari: declares that she has no conflict of interest.
Daniele Altomare: was the recipient of the grant allocated by the European Academy of Neurology (EAN) for data extraction and evidence assessment for the present project.
Federica Agosta: is Section Editor of NeuroImage Clinical; has received speaker fees from Biogen Idec, Novartis, and Excellence in Medical Education; and receives or has received research supports from the Italian Ministry of Health, AriSLA (Fondazione Italiana di Ricerca per la SLA), and the European Research Council. She received personal fees from Elsevier INC.
Stefania Orini:: declares that she has no conflict of interest.
Federica Gandolfo: declares that she has no conflict of interest.
Javier Arbizu: received grants from Eli-Lilly & Co, Piramal and GE Healthcare.
Femke Bouwman: none.
Peter Nestor: none.
Alexander Drzezga: received grants and non-financial support from Eli-Lilly & Co, Siemens, and GE Healthcare; he also received non-financial support from Piramal.
Zuzana Walker: received from GE Healthcare grants and tracers, personal fees for consultancy, and speakers fee.
Marina Boccardi: has received funds from the European Association of Nuclear Medicine (EANM) to perform the evidence assessment and the global coordination of the present project. Moreover, she has received research grants from Piramal, and served as a paid member of advisory boards for Eli Lilly.
Giovanni B Frisoni: is principal investigator of industry-sponsored trials funded by AbbVie, Acadia, Altoida, Amoneta, Araclon, Biogen, Janssen, Novartis, Piramal; has received funding for investigator-initiated trials from GE, Piramal, and Avid-Lilly; has received speaker fees from a number of pharma and imaging companies.
Ethical approval
This is a review article that does not contain any original study with human participants performed by any of the authors. Ethical approval is shown in each of the quoted original paper.
Informed consent
Not applicable, this is a review article. Informed consent statement is declared in each of the revised paper.
Rights and permissions
About this article
Cite this article
Drzezga, A., Altomare, D., Festari, C. et al. Diagnostic utility of 18F-Fluorodeoxyglucose positron emission tomography (FDG-PET) in asymptomatic subjects at increased risk for Alzheimer’s disease. Eur J Nucl Med Mol Imaging 45, 1487–1496 (2018). https://doi.org/10.1007/s00259-018-4032-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4032-1