Abstract
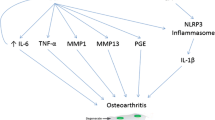
Osteoarthritis (OA) is one of the leading causes of disability worldwide. As our understanding of OA progressively has moved from a purely mechanical “wear and tear” concept toward a complex multi-tissue condition in which inflammation plays a central role, the possible role of crystal-induced inflammation in OA incidence and progression may be relevant. In addition to gout, which affects 4% of the US population, basic calcium phosphate and calcium pyrophosphate deposition both may induce joint inflammation and may play a role in pain in OA. This narrative review article discusses the possible mechanisms underlying the associations between crystal-induced arthropathies and OA, and the important implications of these for clinical practice and future research.



Similar content being viewed by others
References
Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–13.
Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95:986-995.e1.
GBD 2015 Disease and injury incidence and prevalence collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Lond Engl. 2016;388:1545–602.
Abramson SB, Attur M, Yazici Y. Prospects for disease modification in osteoarthritis. Nat Clin Pract Rheumatol. 2006;2:304–12.
Crema MD, Felson DT, Roemer FW, Niu J, Marra MD, Zhang Y, et al. Peripatellar synovitis: comparison between non-contrast-enhanced and contrast-enhanced MRI and association with pain. MOST Study Osteoarthr Cartil. 2013;21:413–8.
Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70:1804–9.
Zhang Y, Nevitt M, Niu J, Lewis C, Torner J, Guermazi A, et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 2011;63:691–9.
Attur M, Krasnokutsky S, Statnikov A, Samuels J, Li Z, Friese O, et al. Low-grade inflammation in symptomatic knee osteoarthritis: prognostic value of inflammatory plasma lipids and peripheral blood leukocyte biomarkers. Arthritis Rheumatol Hoboken NJ. 2015;67:2905–15.
Krasnokutsky S, Belitskaya-Lévy I, Bencardino J, Samuels J, Attur M, Regatte R, et al. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 2011;63:2983–91.
Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr Cartil. 2013;21:16–21.
Neogi T, Krasnokutsky S, Pillinger MH. Urate and osteoarthritis: evidence for a reciprocal relationship. Joint Bone Spine. 2019;86:576–82.
Conway R, McCarthy GM. Calcium-containing crystals and osteoarthritis: an unhealthy alliance. Curr Rheumatol Rep. 2018;20:13.
Zell M, Aung T, Kaldas M, Rosenthal AK, Bai B, Liu T, et al. Calcium pyrophosphate crystal size and characteristics. Osteoarthr Cartil Open. 2021;3:100133.
McCarthy GM, Dunne A. Calcium crystal deposition diseases - beyond gout. Nat Rev Rheumatol. 2018;14:592–602.
Rosenthal AK, Ryan LM. Nonpharmacologic and pharmacologic management of CPP crystal arthritis and BCP arthropathy and periarticular syndromes. Rheum Dis Clin North Am. 2014;40:343–56.
Rosenthal AK. Basic calcium phosphate crystal-associated musculoskeletal syndromes: an update. Curr Opin Rheumatol. 2018;30:168–72.
McCarty DJ, Halverson PB, Carrera GF, Brewer BJ, Kozin F. “Milwaukee shoulder”–association of microspheroids containing hydroxyapatite crystals, active collagenase, and neutral protease with rotator cuff defects. I Clinical aspects. Arthritis Rheum. 1981;24:464–73.
Jones AC, Chuck AJ, Arie EA, Green DJ, Doherty M. Diseases associated with calcium pyrophosphate deposition disease. Semin Arthritis Rheum. 1992;22:188–202.
Doherty M, Watt I, Dieppe PA. Localised chondrocalcinosis in post-meniscectomy knees. Lancet Lond Engl. 1982;1:1207–10.
Ramonda R, Musacchio E, Perissinotto E, Sartori L, Punzi L, Corti MC, et al. Prevalence of chondrocalcinosis in Italian subjects from northeastern Italy. The Pro.V.A. (PROgetto Veneto Anziani) study. Clin Exp Rheumatol. 2009;27:981–4.
Felson DT, Anderson JJ, Naimark A, Kannel W, Meenan RF. The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham Study. J Rheumatol. 1989;16:1241–5.
Guermazi A, Jarraya M, Lynch JA, Felson DT, Clancy M, Nevitt M, et al. Reliability of a new scoring system for intraarticular mineralization of the knee: Boston University Calcium Knee Score (BUCKS). Osteoarthr Cartil. 2020;28:802–10.
Liew J, Guermazi A, Jarraya M, Wang N, Felson D, Lewis CE, et al. The Association of Radiographic Chondrocalcinosis with Localized Structural Outcomes in Knee OA: the multicenter osteoarthritis study [abstract]. Arthritis Rheumatol [Internet]. 2022;74. Available from: https://acrabstracts.org/abstract/the-association-of-radiographic-chondrocalcinosis-with-localized-structural-outcomes-in-knee-oa-the-multicenter-osteoarthritis-study/. Accessed September 17, 2022.
Derfus BA, Kurian JB, Butler JJ, Daft LJ, Carrera GF, Ryan LM, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29:570–4.
Nalbant S, Martinez JAM, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher HR. Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthr Cartil. 2003;11:50–4.
Fuerst M, Bertrand J, Lammers L, Dreier R, Echtermeyer F, Nitschke Y, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009;60:2694–703.
Meyer F, Dittmann A, Kornak U, Herbster M, Pap T, Lohmann CH, et al. Chondrocytes from osteoarthritic and chondrocalcinosis cartilage represent different phenotypes. Front Cell Dev Biol. 2021;9:622287.
Dieppe P, Swan A. Identification of crystals in synovial fluid. Ann Rheum Dis. 1999;58:261–3.
Mitsuyama H, Healey RM, Terkeltaub RA, Coutts RD, Amiel D. Calcification of human articular knee cartilage is primarily an effect of aging rather than osteoarthritis. Osteoarthr Cartil. 2007;15:559–65.
Ea H-K, Nguyen C, Bazin D, Bianchi A, Guicheux J, Reboul P, et al. Articular cartilage calcification in osteoarthritis: insights into crystal-induced stress. Arthritis Rheum. 2011;63:10–8.
Campillo-Gimenez L, Renaudin F, Jalabert M, Gras P, Gosset M, Rey C, et al. Inflammatory potential of four different phases of calcium pyrophosphate relies on NF-κB activation and MAPK pathways. Front Immunol. 2018;9:2248.
Ea H-K, Chobaz V, Nguyen C, Nasi S, van Lent P, Daudon M, et al. Pathogenic role of basic calcium phosphate crystals in destructive arthropathies. PLoS One. 2013;8:e57352.
Molloy ES, Morgan MP, Doherty GA, McDonnell B, O’Byrne J, Fitzgerald DJ, et al. Microsomal prostaglandin E2 synthase 1 expression in basic calcium phosphate crystal-stimulated fibroblasts: role of prostaglandin E2 and the EP4 receptor. Osteoarthr Cartil. 2009;17:686–92.
McCarthy GM, Westfall PR, Masuda I, Christopherson PA, Cheung HS, Mitchell PG. Basic calcium phosphate crystals activate human osteoarthritic synovial fibroblasts and induce matrix metalloproteinase-13 (collagenase-3) in adult porcine articular chondrocytes. Ann Rheum Dis. 2001;60:399–406.
Grandjean-Laquerriere A, Tabary O, Jacquot J, Richard D, Frayssinet P, Guenounou M, et al. Involvement of toll-like receptor 4 in the inflammatory reaction induced by hydroxyapatite particles. Biomater. 2007;28:400–4.
van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthr Cartil. 2012;20:223–32.
Ea HK, Monceau V, Camors E, Cohen-Solal M, Charlemagne D, Lioté F. Annexin 5 overexpression increased articular chondrocyte apoptosis induced by basic calcium phosphate crystals. Ann Rheum Dis. 2008;67:1617–25.
Fam AG, Morava-Protzner I, Purcell C, Young BD, Bunting PS, Lewis AJ. Acceleration of experimental lapine osteoarthritis by calcium pyrophosphate microcrystalline synovitis. Arthritis Rheum. 1995;38:201–10.
Neame RL. UK community prevalence of knee chondrocalcinosis: evidence that correlation with osteoarthritis is through a shared association with osteophyte. Ann Rheum Dis. 2003;62:513–8.
Auw Yang KG, Raijmakers NJH, van Arkel ERA, Caron JJ, Rijk PC, Willems WJ, et al. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthr Cartil. 2008;16:498–505.
Schieker M, Conaghan PG, Mindeholm L, Praestgaard J, Solomon DH, Scotti C, et al. Effects of interleukin-1β inhibition on incident hip and knee replacement : exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2020;173:509–15.
Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–41.
Martillo MA, Nazzal L, Crittenden DB. The crystallization of monosodium urate. Curr Rheumatol Rep. 2014;16:400.
Chhana A, Dalbeth N. The gouty tophus: a review. Curr Rheumatol Rep. 2015;17:19.
Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19:18.
Felson DT. Developments in the clinical understanding of osteoarthritis. Arthritis Res Ther. 2009;11:203.
Simkin PA. The pathogenesis of podagra. Ann Intern Med. 1977;86:230–3.
Fam AG, Stein J, Rubenstein J. Gouty arthritis in nodal osteoarthritis. J Rheumatol. 1996;23:684–9.
Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165:742–8.
Oliveria SA, Felson DT, Cirillo PA, Reed JI, Walker AM. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiol Camb Mass. 1999;10:161–6.
Muehleman C, Li J, Aigner T, Rappoport L, Mattson E, Hirschmugl C, et al. Association between crystals and cartilage degeneration in the ankle. J Rheumatol. 2008;35:1108–17.
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier J-P, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42.
Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41.
Giamarellos-Bourboulis EJ, Mouktaroudi M, Bodar E, van der Ven J, Kullberg B-J, Netea MG, et al. Crystals of monosodium urate monohydrate enhance lipopolysaccharide-induced release of interleukin 1 beta by mononuclear cells through a caspase 1-mediated process. Ann Rheum Dis. 2009;68:273–8.
Zengini E, Hatzikotoulas K, Tachmazidou I, Steinberg J, Hartwig FP, Southam L, et al. Genome-wide analyses using UK Biobank data provide insights into the genetic architecture of osteoarthritis. Nat Genet. 2018;50:549–58.
Major TJ, Dalbeth N, Stahl EA, Merriman TR. An update on the genetics of hyperuricaemia and gout. Nat Rev Rheumatol. 2018;14:341–53.
Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21.
Lai J-H, Luo S-F, Hung L-F, Huang C-Y, Lien S-B, Lin L-C, et al. Physiological concentrations of soluble uric acid are chondroprotective and anti-inflammatory. Sci Rep. 2017;7:2359.
Accart N, Dawson J, Obrecht M, Lambert C, Flueckiger M, Kreider J, et al. Degenerative joint disease induced by repeated intra-articular injections of monosodium urate crystals in rats as investigated by translational imaging. Sci Rep. 2022;12:157.
Roddy E, Zhang W, Doherty M. Are joints affected by gout also affected by osteoarthritis? Ann Rheum Dis. 2007;66:1374–7.
Sun Y, Brenner H, Sauerland S, Günther KP, Puhl W, Stürmer T. Serum uric acid and patterns of radiographic osteoarthritis–the Ulm Osteoarthritis Study. Scand J Rheumatol. 2000;29:380–6.
Roddy E, Zhang W, Doherty M. Gout and nodal osteoarthritis: a case-control study. Rheumatol. 2008;47:732–3.
Kuo C-F, Chou I-J, See L-C, Chen J-S, Yu K-H, Luo S-F, et al. Urate-lowering treatment and risk of total joint replacement in patients with gout. Rheumatol Oxf Engl. 2018;57:2129–39.
Kuo C-F, Grainge MJ, Mallen C, Zhang W, Doherty M. Comorbidities in patients with gout prior to and following diagnosis: case-control study. Ann Rheum Dis. 2016;75:210–7.
Dalbeth N, Aati O, Kalluru R, Gamble GD, Horne A, Doyle AJ, et al. Relationship between structural joint damage and urate deposition in gout: a plain radiography and dual-energy CT study. Ann Rheum Dis. 2015;74:1030–6.
Kravchenko D, Karakostas P, Kuetting D, Meyer C, Brossart P, Behning C, et al. The role of dual energy computed tomography in the differentiation of acute gout flares and acute calcium pyrophosphate crystal arthritis. Clin Rheumatol. 2022;41:223–33.
Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthr Cartil. 2013;21:1474–84.
Le J, Peng Q, Sperling K. Biochemical magnetic resonance imaging of knee articular cartilage: T1rho and T2 mapping as cartilage degeneration biomarkers. Ann N Y Acad Sci. 2016;1383:34–42.
Zhu J, Hu N, Hou J, Liang X, Wang Y, Zhang H, et al. T1rho mapping of cartilage and menisci in patients with hyperuricaemia at 3 T: a preliminary study. Clin Radiol. 2021;76:710.e1-710.e8.
Hu N, Zhu J, Liang X, Wang Y, Guan J, Wen W, et al. T2 MRI at 3T of cartilage and menisci in patients with hyperuricemia: initial findings. Skeletal Radiol. 2022;51:607–18.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AG: received consultancy fees from Pfizer, Novartis, MerckSerono, TissueGene, AstraZeneca, and Regeneron. He is a shareholder of Boston Imaging Core Lab., LLC. FWR: Consultant to Calibr and Grünenthal. He is a shareholder of Boston Imaging Core Lab., LLC. CKK has received consultancy fees from Thuasne, Regeneron, Novartis, Kolon Tissue Gene, Taiwan Liposome, Amzell AZ, LG Chem, Express Scripts, and has received grants from Lilly, Pfizer GSK, Cumberland.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jarraya, M., Roemer, F., Kwoh, C.K. et al. Crystal arthropathies and osteoarthritis—where is the link?. Skeletal Radiol 52, 2037–2043 (2023). https://doi.org/10.1007/s00256-022-04246-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-022-04246-8