Abstract
Objective
To study associations between MRI-derived subchondral trabecular biomarkers obtained from conventional MRI sequences and knee cartilage loss over 12 and 24 months, using the FNIH osteoarthritis (OA) biomarkers consortium.
Materials and methods
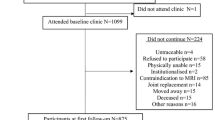
Data of the 600 subjects in the FNIH OA biomarkers consortium (a nested case–control study within Osteoarthritis Initiative [OAI]) were extracted from the online database. Baseline knee MRI (intermediate-weighted (IW) sequences) were evaluated to determine conventional MRI-derived trabecular thickness (cTbTh) and bone-to-total ratio (cBV/TV). The measurements for medial and lateral volumes of cartilages using baseline, 12-, and 24-month knee MRI were extracted from the OAI database, and cartilage volume loss over 12 and 24 months of follow-up were determined using Relative Change Index. The association between conventional MRI-based subchondral trabecular biomarkers and cartilage volume loss were studied using logistic regression models, adjusted for relevant confounders including age, sex, body mass index (BMI), vitamin D use, Kellgren Lawrence grade (KLG), and tibiofemoral alignment.
Results
Higher medial cTbTh and cBV/TV at baseline were associated with increased odds of medial tibial cartilage volume loss over 12 months (ORs: 1.01 [1.00–1.02] and 1.24 [1.10–1.39] per 1-SD change) and 24 months (ORs: 1.01 [1.00–1.02] and 1.22 [1.08–1.37], per 1-SD change). No significant association was observed between medial subchondral trabecular biomarkers and lateral tibial or femoral (medial or lateral) cartilage volume loss over the first and second follow-up years.
Conclusions
Conventional MRI-derived subchondral trabecular biomarkers (higher medial cTbTh and cBV/TV) may be associated with increased medial tibial cartilage volume loss as early as 1 year.


Similar content being viewed by others
References
Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2):160–7.
Hochberg MC, Guermazi A, Guehring H, Aydemir A, Wax S, Fleuranceau-Morel P, et al. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: the FORWARD randomized clinical trial. JAMA. 2019;322(14):1360–70.
Zhang W, McWilliams DF, Ingham SL, Doherty SA, Muthuri S, Muir KR, et al. Nottingham knee osteoarthritis risk prediction models. Ann Rheum Dis. 2011;70(9):1599–604.
Roos EM, Arden NK. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol. 2016;12(2):92–101.
Pishgar F, Guermazi A, Roemer FW, Link TM, Demehri S. Conventional MRI-based subchondral trabecular biomarkers as predictors of knee osteoarthritis progression: data from the Osteoarthritis Initiative. Eur Radiol. 2020:1–10.
Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–9.
Wluka AE, Wang Y, Davies-Tuck M, English DR, Giles GG, Cicuttini FM. Bone marrow lesions predict progression of cartilage defects and loss of cartilage volume in healthy middle-aged adults without knee pain over 2 yrs. Rheumatology (Oxford). 2008;47(9):1392–6.
MacKay JW, Kapoor G, Driban JB, Lo GH, McAlindon TE, Toms AP, et al. Association of subchondral bone texture on magnetic resonance imaging with radiographic knee osteoarthritis progression: data from the Osteoarthritis Initiative Bone Ancillary Study. Eur Radiol. 2018;28(11):4687–95.
Chiba K, Uetani M, Kido Y, Ito M, Okazaki N, Taguchi K, et al. Osteoporotic changes of subchondral trabecular bone in osteoarthritis of the knee: a 3-T MRI study. Osteoporos Int. 2012;23(2):589–97.
MacKay JW, Murray PJ, Kasmai B, Johnson G, Donell ST, Toms AP. Subchondral bone in osteoarthritis: association between MRI texture analysis and histomorphometry. Osteoarthritis Cartilage. 2017;25(5):700–7.
Marques J, Genant HK, Lillholm M, Dam EB. Diagnosis of osteoarthritis and prognosis of tibial cartilage loss by quantification of tibia trabecular bone from MRI. Magn Reson Med. 2013;70(2):568–75.
Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–7.
Neogi T, Nevitt M, Niu J, Sharma L, Roemer F, Guermazi A, et al. Subchondral bone attrition may be a reflection of compartment-specific mechanical load: the MOST Study. Ann Rheum Dis. 2010;69(5):841–4.
Castañeda S, Roman-Blas JA, Largo R, Herrero-Beaumont G. Subchondral bone as a key target for osteoarthritis treatment. Biochem Pharmacol. 2012;83(3):315–23.
Hafezi-Nejad N, Guermazi A, Roemer FW, Hunter DJ, Dam EB, Zikria B, et al. Prediction of medial tibiofemoral compartment joint space loss progression using volumetric cartilage measurements: data from the FNIH OA biomarkers consortium. Eur Radiol. 2017;27(2):464–73.
Haj-Mirzaian A, Guermazi A, Pishgar F, Roemer FW, Sereni C, Hakky M, et al. Patellofemoral morphology measurements and their associations with tibiofemoral osteoarthritis-related structural damage: exploratory analysis on the osteoarthritis initiative. Eur Radiol. 2020;30(1):128–40.
Hunter D, Nevitt M, Lynch J, Kraus VB, Katz JN, Collins JE, et al. Longitudinal validation of periarticular bone area and 3D shape as biomarkers for knee OA progression? Data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2016;75(9):1607–14.
Culvenor AG, Engen CN, Øiestad BE, Engebretsen L, Risberg MA. Defining the presence of radiographic knee osteoarthritis: a comparison between the Kellgren and Lawrence system and OARSI atlas criteria. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3532–9.
Sezgin M, Sankur B. Survey over image thresholding techniques and quantitative performance evaluation. J Electron Imaging. 2004;13(1):146–66.
Doube M, Kłosowski MM, Arganda-Carreras I, Cordelières FP, Dougherty RP, Jackson JS, et al. BoneJ: Free and extensible bone image analysis in ImageJ. Bone. 2010;47(6):1076–9.
Dam EB, Lillholm M, Marques J, Nielsen M. Automatic segmentation of high- and low-field knee MRIs using knee image quantification with data from the osteoarthritis initiative. J Med Imaging (Bellingham). 2015;2(2):024001.
Haj-Mirzaian A, Guermazi A, Hafezi-Nejad N, Sereni C, Hakky M, Hunter DJ, et al. Superolateral Hoffa’s fat pad (SHFP) oedema and patellar cartilage volume loss: quantitative analysis using longitudinal data from the Foundation for the National Institute of Health (FNIH) Osteoarthritis Biomarkers Consortium. Eur Radiol. 2018;28(10):4134–45.
Wise EA. Methods for analyzing psychotherapy outcomes: a review of clinical significance, reliable change, and recommendations for future directions. J Pers Assess. 2004;82(1):50–9.
Kraus VB, Collins JE, Charles HC, Pieper CF, Whitley L, Losina E, et al. Predictive validity of radiographic trabecular bone texture in knee osteoarthritis: the Osteoarthritis Research Society International/Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol (Hoboken, NJ). 2018;70(1):80–7.
Lo GH, Schneider E, Driban JB, Price LL, Hunter DJ, Eaton CB, et al. Periarticular bone predicts knee osteoarthritis progression: data from the Osteoarthritis Initiative. Semin Arthritis Rheum. 2018;48(2):155–61.
Tanamas SK, Wluka AE, Pelletier JP, Pelletier JM, Abram F, Berry PA, et al. Bone marrow lesions in people with knee osteoarthritis predict progression of disease and joint replacement: a longitudinal study. Rheumatology (Oxford). 2010;49(12):2413–9.
Liu C, Liu C, Ren X, Si L, Shen H, Wang Q, et al. Quantitative evaluation of subchondral bone microarchitecture in knee osteoarthritis using 3T MRI. BMC Musculoskelet Disord. 2017;18(1):496.
Liu C, Liu C, Si L, Shen H, Wang Q, Yao W. Relationship between subchondral bone microstructure and articular cartilage in the osteoarthritic knee using 3T MRI. J Magn Reson Imaging: JMRI. 2018.
Crema MD, Cibere J, Sayre EC, Roemer FW, Wong H, Thorne A, et al. The relationship between subchondral sclerosis detected with MRI and cartilage loss in a cohort of subjects with knee pain: the knee osteoarthritis progression (KOAP) study. Osteoarthritis Cartilage. 2014;22(4):540–6.
Chappard C, Peyrin F, Bonnassie A, Lemineur G, Brunet-Imbault B, Lespessailles E, et al. Subchondral bone micro-architectural alterations in osteoarthritis: a synchrotron micro-computed tomography study. Osteoarthritis Cartilage. 2006;14(3):215–23.
Yuan XL, Meng HY, Wang YC, Peng J, Guo QY, Wang AY, et al. Bone-cartilage interface crosstalk in osteoarthritis: potential pathways and future therapeutic strategies. Osteoarthritis Cartilage. 2014;22(8):1077–89.
Findlay DM, Atkins GJ. Osteoblast-chondrocyte interactions in osteoarthritis. Curr Osteoporos Rep. 2014;12(1):127–34.
Wang Y, Wluka AE, Cicuttini FM. The determinants of change in tibial plateau bone area in osteoarthritic knees: a cohort study. Arthritis Res Ther. 2005;7(3):R687.
Beuf O, Ghosh S, Newitt DC, Link TM, Steinbach L, Ries M, et al. Magnetic resonance imaging of normal and osteoarthritic trabecular bone structure in the human knee. Arthritis Rheum. 2002;46(2):385–93.
Ding C, Cicuttini F, Jones G. Tibial subchondral bone size and knee cartilage defects: relevance to knee osteoarthritis. Osteoarthritis Cartilage. 2007;15(5):479–86.
Dore D, Martens A, Quinn S, Ding C, Winzenberg T, Zhai G, et al. Bone marrow lesions predict site-specific cartilage defect development and volume loss: a prospective study in older adults. Arthritis Res Ther. 2010;12(6):R222.
Hunter DJ, Gerstenfeld L, Bishop G, Davis AD, Mason ZD, Einhorn TA, et al. Bone marrow lesions from osteoarthritis knees are characterized by sclerotic bone that is less well mineralized. Arthritis Res Ther. 2009;11(1):R11.
Roemer FW, Jarraya M, Niu J, Duryea J, Lynch JA, Guermazi A. Knee joint subchondral bone structure alterations in active athletes: a cross-sectional case-control study. Osteoarthritis Cartilage. 2015;23(12):2184–90.
Acknowledgements
The OAI was a public-private partnership comprised of several contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health (NIH), a branch of the Department of Health and Human Services, and conducted by the Osteoarthritis Initiative (OAI) Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public-use dataset and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
Moreover, several grants and direct or in-kind contributions provide the publicly available data from the FNIH OA Biomarkers Consortium, including AbbVie, Amgen, Arthritis Foundation, Artialis; Bioiberica, BioVendor, DePuy, Flexion Therapeutics, GSK, IBEX, IDS, Merck Serono, Quidel, Rottapharm | Madaus, Sanofi, Stryker, the Pivotal OAI MRI Analyses (POMA) study, NIH HHSN2682010000 21C, and the Osteoarthritis Research Society International.
The summary of data related to this study has been presented as a congress abstract (available at https://doi.org/10.1016/j.joca.2021.02.126).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pishgar, F., Ashraf-ganjouei, A., Dolatshahi, M. et al. Conventional MRI-derived subchondral trabecular biomarkers and their association with knee cartilage volume loss as early as 1 year: a longitudinal analysis from Osteoarthritis Initiative. Skeletal Radiol 51, 1959–1966 (2022). https://doi.org/10.1007/s00256-022-04042-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-022-04042-4