Abstract
Objective
To develop and evaluate deep learning (DL) risk assessment models for predicting pain progression in subjects with or at risk of knee osteoarthritis (OA).
Materials and methods
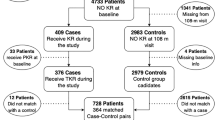
The incidence and progression cohorts of the Osteoarthritis Initiative, a multi-center longitudinal study involving 9348 knees in 4674 subjects with or at risk of knee OA that began in 2004 and is ongoing, were used to conduct this retrospective analysis. A subset of knees without and with pain progression (defined as a 9-point or greater increase in pain score between baseline and two or more follow-up time points over the first 48 months) was randomly stratified into training (4200 knees with a mean age of 61.0 years and 60% female) and hold-out testing (500 knees with a mean age of 60.8 years and 60% female) datasets. A DL model was developed to predict pain progression using baseline knee radiographs. An artificial neural network was used to develop a traditional risk assessment model to predict pain progression using demographic, clinical, and radiographic risk factors. A combined model was developed to combine demographic, clinical, and radiographic risk factors with DL analysis of baseline knee radiographs. Area under the curve (AUC) analysis was performed using the hold-out testing dataset to evaluate model performance.
Results
The traditional model had an AUC of 0.692 (66.9% sensitivity and 64.1% specificity). The DL model had an AUC of 0.770 (76.7% sensitivity and 70.5% specificity), which was significantly higher (p < 0.001) than the traditional model. The combined model had an AUC of 0.807 (72.3% sensitivity and 80.9% specificity), which was significantly higher (p < 0.05) than the traditional and DL models.
Conclusions
DL models using baseline knee radiographs had higher diagnostic performance for predicting pain progression than traditional models using demographic, clinical, and radiographic risk factors.








Similar content being viewed by others
References
Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38(10):1500–5.
Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr Cart. 2013;21(9):1145–53.
Neogi T, Frey-Law L, Scholz J, Niu J, Arendt-Nielsen L, Woolf C, et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis. 2015;74(4):682–8.
Sheeran P, Harris PR, Epton T. Does heightening risk appraisals change people’s intentions and behavior? A meta-analysis of experimental studies. Psychol Bull. 2014;140(2):511–43.
Roddy E, Doherty M. Changing life-styles and osteoarthritis: what is the evidence? Best Pract Res Clin Rheumatol. 2006;20(1):81–97.
Nguyen C, Lefevre-Colau MM, Poiraudeau S, Rannou F. Evidence and recommendations for use of intra-articular injections for knee osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):184–9.
Hong T, Wang H, Li G, Yao P, Ding Y. Systematic review and meta-analysis of 12 randomized controlled trials evaluating the efficacy of invasive radiofrequency treatment for knee pain and function. Biomed Res Int. 2019;2019:9037510.
Amendola A, Bonasia DE. Results of high tibial osteotomy: review of the literature. Int Orthop. 2010;34(2):155–60.
Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27(6):1513–7.
Hochberg MC, Lawrence RC, Everett DF, Cornoni-Huntley J. Epidemiologic associations of pain in osteoarthritis of the knee: data from the National Health and Nutrition Examination Survey and the National Health and Nutrition Examination-I Epidemiologic Follow-up Survey. Semin Arthritis Rheum. 1989;18(4 Suppl 2):4–9.
Lethbridge-Cejku M, Scott WW Jr, Reichle R, Ettinger WH, Zonderman A, Costa P, et al. Association of radiographic features of osteoarthritis of the knee with knee pain: data from the Baltimore Longitudinal Study of Aging. Arthritis Care Res. 1995;8(3):182–8.
Paradowski PT, Englund M, Lohmander LS, Roos EM. The effect of patient characteristics on variability in pain and function over two years in early knee osteoarthritis. Health Qual Life Outcomes. 2005;3:59.
Jinks C, Jordan KP, Blagojevic M, Croft P. Predictors of onset and progression of knee pain in adults living in the community. A prospective study. Rheumatology. 2008;47(3):368–74.
Mallen CD, Peat G, Thomas E, Lacey R, Croft P. Predicting poor functional outcome in community-dwelling older adults with knee pain: prognostic value of generic indicators. Ann Rheum Dis. 2007;66(11):1456–61.
Collins JE, Katz JN, Dervan EE, Losina E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the Osteoarthritis Initiative. Osteoarthr Cartil. 2014;22(5):622–30.
Vina ER, Ran D, Ashbeck EL, Kwoh CK. Natural history of pain and disability among African-Americans and whites with or at risk for knee osteoarthritis: a longitudinal study. Osteoarthr Cartil. 2018;26(4):471–9.
Oak SR, Ghodadra A, Winalski CS, Miniaci A, Jones MH. Radiographic joint space width is correlated with 4-year clinical outcomes in patients with knee osteoarthritis: data from the Osteoarthritis Initiative. Osteoarthr Cartil. 2013;21(9):1185–90.
Halilaj E, Le Y, Hicks JL, Hastie TJ, Delp SL. Modeling and predicting osteoarthritis progression: data from the Osteoarthritis Initiative. Osteoarthr Cartil. 2018;26(12):1643–50.
Landsmeer MLA, Runhaar J, van Middelkoop M, Oei EHG, Schiphof D, Bindels PJE, et al. Predicting knee pain and knee osteoarthritis among overweight women. J Am Board Fam Med. 2019;32(4):575–84.
Urish KL, Keffalas MG, Durkin JR, Miller DJ, Chu CR, Mosher TJ. T2 texture index of cartilage can predict early symptomatic OA progression: data from the Osteoarthritis Initiative. Osteoarthr Cartil. 2013;21(10):1550–7.
Zhong H, Miller DJ, Urish KL. T2 map signal variation predicts symptomatic osteoarthritis progression: data from the Osteoarthritis Initiative. Skelet Radiol. 2016;45(7):909–13.
Singh SP, Wang L, Gupta S, Goli H, Padmanabhan P, Gulyas B. 3D deep learning on medical images: a review. Sensors (Basel). 2020;20(18).
Chang GH, Felson DT, Qiu S, Guermazi A, Capellini TD, Kolachalama VB. Assessment of knee pain from MR imaging using a convolutional Siamese network. Eur Radiol. 2020;30(6):3538–48.
Tiulpin A, Klein S, Bierma-Zeinstra SMA, Thevenot J, Rahtu E, Meurs JV, et al. Multimodal machine learning-based knee osteoarthritis progression prediction from plain radiographs and clinical data. Sci Rep. 2019;9(1):20038.
Guan B, Liu F, Haj-Mirzaian A, Demehri S, Samsonov A, Neogi T, et al. Deep learning risk assessment models for predicting progression of radiographic medial joint space loss over a 48-month follow-up period. Osteoarthr Cartil. 2020;28(4):428–37.
Leung K, Zhang B, Tan J, Shen Y, Geras KJ, Babb JS, et al. Prediction of total knee replacement and diagnosis of osteoarthritis by using deep learning on knee radiographs: data from the Osteoarthritis Initiative. Radiology. 2020;296(3):584–93.
Tolpadi AA, Lee JJ, Pedoia V, Majumdar S. Deep learning predicts total knee replacement from magnetic resonance images. Sci Rep. 2020;10(1):6371.
Lester G. Clinical research in OA--the NIH Osteoarthritis Initiative. J Musculoskelet Neuronal Interact. 2008;8(4):313–4.
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502.
Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29(12):2473–6.
FNIH. Osteoarthritis Biomarkers Consortium FNIH Project: study design. https://www.oai.ucsf.edu/datarelease/biospecimens.asp. Accessed June 20, 2019.
Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384–91.
Hafezi-Nejad N, Guermazi A, Roemer FW, Hunter DJ, Dam EB, Zikria B, et al. Prediction of medial tibiofemoral compartment joint space loss progression using volumetric cartilage measurements: data from the FNIH OA biomarkers consortium. Eur Radiol. 2017;27(2):464–73.
Redmon J, Divvala S, Girshick R, Farhadi A. You Only Look Once: unified, real-time object detection. Proc CVPR IEEE. 2016:779–88.
Deng J, Dong W, Socher R, Li LJ, Li K, Li FF. ImageNet: a large-scale hierarchical image database. CVPR: 2009 IEEE Conference on Computer Vision and Pattern Recognition. 2009;1–4:248–55.
Huang G, Liu Z, van der Maaten L, Weinberger KQ. Densely connected convolutional networks. 30th IEEE Conference on Computer Vision and Pattern Recognition (CVPR 2017). 2017:2261–9.
Tan M, Le Q. EfficientNet: rethinking model scaling for convolutional neural networks. In: Kamalika C, Ruslan S, editors. Proceedings of the 36th international conference on machine learning: Proceedings of Machine Learning Research: PMLR; 2019. p. 6105–14.
Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458–72.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45.
Funding
Funding support for the manuscript was provided by the National Institute of Arthritis and Musculoskeletal and Skin Disease R01-AR068373-01 grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Guan, B., Liu, F., Mizaian, A.H. et al. Deep learning approach to predict pain progression in knee osteoarthritis. Skeletal Radiol 51, 363–373 (2022). https://doi.org/10.1007/s00256-021-03773-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-021-03773-0