Abstract
Galacto-oligosaccharides (GOS) are prebiotic compounds that are mainly used in infant formula to mimic bifidogenic effects of mother’s milk. They are synthesized by β-galactosidase enzymes in a trans-glycosylation reaction with lactose. Many β-galactosidase enzymes from different sources have been studied, resulting in varying GOS product compositions and yields. The in vivo role of these enzymes is in lactose hydrolysis. Therefore, the best GOS yields were achieved at high lactose concentrations up to 60%wt, which require a relatively high temperature to dissolve. Some thermostable β-glucosidase enzymes from thermophilic bacteria are also capable of using lactose or para nitrophenyl-galactose as a substrate. Here, we describe the use of the β-glucosidase BglA from Thermotoga maritima for synthesis of oligosaccharides derived from lactose and cellobiose and their detailed structural characterization. Also, the BglA enzyme kinetics and yields were determined, showing highest productivity at higher lactose and cellobiose concentrations. The BglA trans-glycosylation/hydrolysis ratio was higher with 57%wt lactose than with a nearly saturated cellobiose (20%wt) solution. The yield of GOS was very high, reaching 72.1%wt GOS from lactose. Structural elucidation of the products showed mainly β(1 → 3) and β(1 → 6) elongating activity, but also some β(1 → 4) elongation was observed. The β-glucosidase BglA from T. maritima was shown to be a very versatile enzyme, producing high yields of oligosaccharides, particularly GOS from lactose.
Key points
• β-Glucosidase of Thermotoga maritima synthesizes GOS from lactose at very high yield.
• Thermotoga maritima β-glucosidase has high activity and high thermostability.
• Thermotoga maritima β-glucosidase GOS contains mainly (β1-3) and (β1-6) linkages.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Galacto-oligosaccharides (GOS) are synthesized from lactose via a trans-glycosylation reaction catalyzed by normally hydrolytic β-galactosidase enzymes (e.g., the Bacillus circulans β-galactosidase BgaD). These β-galactosidase enzymes belong to the glycoside hydrolase (GH) A class of glycoside hydrolase enzymes (www.CAZy.org), containing a TIM (β/α)8 barrel catalytic domain (Drula et al. 2022). Within this class, there are several families, based on amino acid sequence similarity. GH-A clan β-galactosidases (EC 3.2.1.23) belong to families GH1, 2, 35, 42, 50, 59, and 147. Various studies have identified a wide variety of structures in commercial GOS products (van Leeuwen et al. 2016), based on effective GOS-synthesizing enzymes (Nguyen et al. 2006; Cardelle-Cobas et al. 2008; Goulas et al. 2009; Rodriguez-Colinas et al. 2011; Yin et al. 2017a).
Not all compounds that are currently considered to have prebiotic properties pass through the mammalian digestive system entirely intact (Bindels et al. 2015; Yan et al. 2018). Using rat intestinal extracts, a commercial GOS mixture was hydrolyzed up to 50% (Ferreira-Lazarte et al. 2017). Particularly, GOS with a degree of polymerization (DP) of 2 and 3 were susceptible to hydrolysis. Still, addition of GOS to infant nutrition has been shown to shift the gut microbiome towards a higher amount of bifidobacteria in vivo (Oozeer et al. 2013). Also, in studies with adults and livestock, GOS bifidogenic activity has been observed (Jung et al. 2008; Davis et al. 2010; Vulevic et al. 2015; Alizadeh et al. 2016). Previous work has suggested that β-galactosidases from bifidobacteria have a preference for hydrolyzing β(1 → 6) and β(1 → 3) linkages (Cardelle-Cobas et al. 2011; Arreola et al. 2014), resulting in release of glucose and galactose as carbon sources for growth, rendering such GOS the most suitable for use in infant nutrition.
Most β-galactosidase enzymes that have a preference for the synthesis of β(1 → 3) or β(1 → 6)-linked GOS from lactose show only moderate yields (27–44%wt) (Arreola et al. 2014; Kittibunchakul et al. 2020; Füreder et al. 2020). The most commonly used commercial enzyme, B. circulans BgaD enzyme, reaches yields up to 63.3%wt (Benjamins et al. 2014). The BgaD enzyme, however, produces mainly β(1 → 4)-linkages (Van Leeuwen et al. 2014). Therefore, we are evaluating other atypical β-galactosidase enzymes for the synthesis of novel GOS mixtures, reaching high GOS yields with predominantly β(1 → 3) and β(1 → 6) linkages.
Glucose oligosaccharides, containing β-linked residues, are less well studied, but early studies have shown promising properties (Rycroft et al. 2001; Kothari and Goyal 2015; Sahasrabudhe et al. 2016; Lee et al. 2020; Yu et al. 2023). Cello-oligosaccharides containing β(1 → 4)-linked glucose units are resistant to digestion, but are also not used by the gut microbiota (Yu et al. 2023). In a preliminary study, gentio-oligosaccharides, consisting of β(1 → 6)-linked glucose units, were shown to have prebiotic properties (Rycroft et al. 2001; Kothari and Goyal 2015). β-Gluco-oligosaccharides with β(1 → 3)-linked residues showed potential as prebiotic, but also stimulated the immune system (Sahasrabudhe et al. 2016; Yu et al. 2023). Most of these oligosaccharides are currently produced by partial acid hydrolysis or enzymatic hydrolysis of natural polysaccharides, like curdlan and laminarin (Sahasrabudhe et al. 2016; Lee et al. 2020; Yu et al. 2023).
A few studies have shown that β-glucosidase enzymes from extremophilic bacterial species are also capable of hydrolyzing lactose (Gabelsberger et al. 1993), even performing trans-glycosylation reactions (Goyal et al. 2001). Recently, the GOS product synthesized from lactose by the β-glucosidase from Thermotoga naphthophila was suggested to contain only one structure, i.e., 3′-galactosyllactose (Yang et al. 2018). Here, we report the analysis of the trans-glycosylation activity of the T. maritima–derived GH2 β-glucosidase (rTmBglA), using lactose and cellobiose as single substrates (both as donor and acceptor). rTmBglA displayed a highly effective trans-glycosylation activity with lactose (GOS; yield 411 g/L; 72.1%wt) and cellobiose (GlcOS; yield 115 g/L; 57.7%wt). Structural characterization of the trans-glycosylase product mixtures showed that with lactose and cellobiose, the rTmBglA enzyme had a preference for formation of β(1 → 3) and β(1 → 6) linkages. Lower levels of β(1 → 4) elongated products were also observed with both substrates, while higher relative β(1 → 4) activity was observed in the glucosyltransferase reaction with cellobiose than in the galactosyltransferase reaction with lactose.
Materials and methods
Materials
The T. maritima β-glucosidase A (TmBglA) was purchased from Megazyme Int (prod. nr. E-BGOSTM).
Expression vector pET100/D-TOPO-rTmBglA, containing the synthetic, recombinant rTmBglA encoding gene (446 aa sequence from UniProtBK accession number Q08638, BglA gene from T. maritima strain ATCC 43589/DSM 3109/JCM 10099/NBRC 100826/MSB, with added His6-tag; Supporting information contains full nucleotide sequence), was purchased from GeneArt (ThermoScientific) and transformed into Ca2+ competent E. coli BL21 star (DE3) cells by heat-shock treatment.
Enzyme expression
E. coli BL21 star (DE3) containing the pET100/D-TOPO-rTmGblA plasmid was grown in 100-mL LB medium, containing 100 μg/mL ampicillin to an optical density (600 nm) of 0.75. The expression of the rTmBglA encoding gene was induced by isopropyl-β-d-thiogalactopyranoside (IPTG; 0.1 mg/mL final concentration), and cells were cultivated overnight at 17 °C. Cells were harvested by centrifugation (10,000 × g, 20 min) and subjected to chemical lysis using B-PER (Thermo Scientific, Pierce), containing lysozyme. Cell debris was centrifuged (15,000 × g, 20 min), and cell-free extract was subjected to heat treatment (75 °C, 10 min) to precipitate thermo-labile E. coli proteins, followed by centrifugation (10,000 × g, 20 min). The clear supernatant was used to further purify rTmGblA by Ni2+ affinity chromatography on Ni–NTA (Sigma-Aldrich). Protein was bound to the Ni–NTA column during incubation for 4 h at 4 °C. After washing the column with 25 mM Tris–HCl (pH 8.0) containing 1 mM CaCl2, protein was eluted with the same buffer containing 200 mM imidazole. The enzyme was desalted and concentrated with a 30 kDa cut-off centrifugal filter and reconstituted to 0.5-mL final volume in Milli-Q water. Protein concentration was determined in six repeats by Nanodrop 2000 spectrophotometer (Isogen Life Science) analysis in relation to a BSA calibration curve, using Milli-Q water as blank. Purity was verified by SDS-PAGE analysis.
Enzyme reactions
Enzyme kinetics towards lactose and cellobiose was analyzed with the glucose oxidase/peroxidase assay (GOPOD), measuring glucose release to determine total units of activity (U). For lactose, 1 U of activity was defined as 1 μmol Glc released per min by 1 mg of enzyme. In case of cellobiose, one cycle of activity will release 2 Glc units; thus, 1 U is equal to the release of 2 μmol Glc per min by 1 mg of enzyme. Kinetic properties with lactose were analyzed in triplicate (n = 3), using a concentration range of 0.1–75 mM lactose incubated with 3.38 µg of rTmBglA enzyme in 150-µL incubations. Kinetics with cellobiose was analyzed with two triplicates (n = 6), using a concentration range of 1–250 mM cellobiose incubated with 3.38 µg of rTmBglA enzyme in 150-µL incubations.
Initial optimization experiments for GOS synthesis were performed with TmBglA obtained from Megazyme, using varying lactose concentrations (45 and 75%wt), pH (5 and 8.5), temperatures (60 or 90 °C), and enzyme units (2.5 or 5 U/g lactose). As a reference, incubation according to typical industrial production (57%wt lactose, 75 °C, pH 6.8, using 3.75 U/g lactose) was also taken along. It should be noted that lactose slurries were prepared by weighing 450 mg lactose and adding 550 μL buffer, 570 mg lactose, adding 430 μL buffer or 750 mg lactose, adding 250 μL buffer and heating to 90 °C for 10 min allowing slurry formation, followed by cooling to the desired reaction temperature. Subsequent optimization was performed with the purified synthetic rTmBglA gene product, using 3.75 U/g lactose and 57%wt lactose and varying pH (from 3 to 9) and temperatures (from 60 to 90 °C), to determine optimum pH and temperature values. Yield optimization experiments were performed using the previously determined reaction optima, with lactose concentrations varying from 50 to 65%wt lactose, prepared as described above. Final GOS synthesis at optimal yield was performed using 3.75 U/g lactose, with 57%wt lactose at 75 °C in a 25 mM NaOAc buffer (pH 5.5). Incubations with 20%wt cellobiose were performed at the same temperature and pH conditions, using the same amount of enzyme (3.75 β-galactosidase U/g substrate). Activity and GOS yields were monitored by profiling of galactose (Gal), glucose (Glc), and lactose against 5-point standard curves (80–1000 μM) using high performance anion exchange chromatography (HPAEC) coupled with a pulsed amperometric detector (PAD). GOS yields were determined by the difference between initial lactose and remaining lactose, corrected for hydrolysis derived from Gal formation.
NMR spectroscopy
Product samples were lyophilized and exchanged twice with 99.9 atom% D2O (Cambridge Isotope Laboratories, Inc.). Finally, samples were dissolved in 650 μL D2O, containing internal acetone (δ1H 2.225). One-dimensional 1H NMR spectra were recorded on a Varian Inova 600 MHz spectrometer (NMR Department, University of Groningen) at a probe temperature of 25 °C. Spectra were recorded in 16–32 transients of 16 k data points and a sweep width of 4800 Hz. After zero filling to 32 k and Fourier transform, the spectra were phased manually, and a Whittaker Smoother baseline correction was applied in MestReNova 10.0.02 (MestRelabs, Santiago de Compostella, Spain).
HPAEC-PAD
GOS and GlcOS incubations are diluted to 0.5 mg/mL initial substrate. Injections of 10-μL diluted GOS and GlcOS products, as well as five quantitative calibration standards, containing Gal, Glc, cellobiose, and lactose in a concentration range of 75 μM up to 1 mM, were profiled on a Dionex ICS-3000 workstation (Dionex, Amsterdam, The Netherlands), equipped with a CarboPac PA-1 column (250 × 2 mm, Dionex) and an ICS-3000 ED pulsed amperometric detector (PAD), using a complex gradient of A: 100 mM NaOH, B: 600 mM NaOAc in 100 mM NaOH, C: Milli-Q water, and D: 50 mM NaOAc. The fractionations were performed at 0.25 mL/min with 10% A, 85% C, and 5% D in 25 min to 40% A, 10% C, and 50% D, followed by a 35-min gradient to 75% A, 25% B, directly followed by 5-min washing with 100% B and reconditioning for 7 min with 10% A, 85% B, and 5% D.
Results
Enzyme purity assessment
The Megazyme enzyme was analyzed by SDS-PAGE to check purity (Supplementary Fig. S1A). Besides a strong band at the expected MW of ~ 52 kDa, bands of smaller proteins were observed in the preparation. An expression-vector plasmid containing a synthetic gene (GeneArt; Thermo Scientific) encoding rTmBglA (Q08638 with His6-tag) was purchased and successfully transferred into an expression host E. coli BL21 (DE3*) and expressed by IPTG induction. Purification with Ni–NTA affinity chromatography did not yield completely pure protein (Supplementary Fig. S1B); therefore, a heat-treatment step (75 °C, 10 min) followed by centrifugation to precipitate denatured protein prior to the Ni–NTA step was added, showing only one protein band on SDS-PAGE (Supplementary Fig. S1C).
The purified recombinant enzyme concentration was determined by UV280 nm absorption on a Nanodrop machine, relative to a BSA standard curve. From 100-mL E. coli culture expression, 3.38-mg active, pure rTmBglA enzyme was obtained. The purified rTmBglA protein was used for kinetic analysis with lactose and cellobiose as substrates and for GOS and GlcOS synthesis.
Optimization experiments
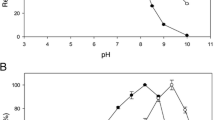
HPAEC-PAD (Fig. 1) profiling of the incubation with lactose showed Glc and Gal release plus a broad range of lactose-derived oligosaccharides. Using the commercial Megazyme TmBglA preparation, several variations in conditions were explored: pH (5 and 8.5), temperature (60 and 90 °C), substrate concentrations (45 and 75%wt), and enzyme amounts (2.50 and 5.00 β-galactosidase U/g lactose). As a comparison, also conditions more similar to those used for GOS synthesis with the Bacillus circulans β-galactosidase BgaD were used, i.e., 57%wt lactose, 75 °C, pH 6.8 with 3.75 β-galactosidase U/g lactose (Yin et al. 2017a). It should be noted that for B. circulans, BgaD reactions were performed at 65 °C, due to loss of activity at higher temperatures. HPAEC-PAD profiles (not shown) showed different levels of lactose utilized and different levels of free Gal produced, which indicated differences in trans-glycosylation/hydrolysis ratios. These initial chromatograms were used to determine optimal conditions. Visual observations (Supplementary Fig. S2) showed strong browning of the reaction mixtures incubated at 90 °C. Reaction mixtures with 75%wt lactose turned darker than reactions using 45%wt lactose under the same conditions.
Further optimization was performed using the recombinant rTmBglA at 75 °C. Using HPAEC-PAD quantitation against standard curves of Gal, Glc, lactose, and cellobiose (not shown), yields of GOS and GlcOS could be determined. The highest total GOS production (~ 432 g/L) was obtained with 65%wt lactose and highest relative GOS yields (~ 0.68 g GOS/g lactose) at 57%wt lactose. Using 57%wt lactose at 75 °C, the enzyme showed a broad pH optimum between pH 5.2 and pH 7.0 (Fig. 2). Maximum yield experiments were performed at pH 6.8, using 57%wt lactose incubated at 75 °C with 3.75 U/g lactose (72.1%wt yield) and 10.0 U/g lactose (63.3%wt yield) enzyme activity added (Table 1). Incubation of rTmBglA with cellobiose (22 g/L) at 75 °C using the 3.75 U/g cellobiose resulted in a maximum yield of 57.7%wt GlcOS (Table 1).
Enzyme kinetics
The maximum enzyme-specific activity (Vmax) and the Michaelis–Menten constant (Km) of rTmBglA were determined for lactose and cellobiose using the GOPOD assay for measuring Glc release after 5-min reaction at different substrate concentrations, using Lineweaver–Burk plots (Table 2). The kinetics of the enzyme with lactose showed a biphasic Lineweaver–Burk plot with two separate sets of catalytic parameters (Supplementary Fig. S3). With cellobiose as substrate, the Vmax was 63.1 µmol/min/mg enzyme and Km 22.3 mM. With lactose at low concentration, Vmax was 1.6 µmol/min/mg while at high-substrate concentrations, 22.1 µmol/min/mg was observed. The Km values with lactose at low- and high-substrate concentrations were 0.4 and 12.9 mM, respectively.
GOS characterization
MALDI-TOF–MS analysis of the products synthesized from lactose (Fig. 3A) showed peaks at 365, 527, 689, and 851 m/z, fitting sodium adducts of di-, tri-, tetra-, and penta-hexose structures, respectively. The HPAEC-PAD profile of maximum yield GOS mixture (Fig. 1) showed a complex peak pattern that was annotated based on retention times in comparison with previously studied GOS mixtures (van Leeuwen et al. 2016; Yin et al. 2017a), combined with NMR spectroscopy data (Fig. 4). NMR structural-reporter-group signals showed specific peaks, supporting the structures identified from the HPAEC-PAD elution times (Table 3; Fig. 5). The main non-lactose DP2 peak corresponded with β-d-Galp-(1 → 3)-d-Glc and β-d-Galp-(1 → 2)- d-Glc. The trisaccharide peak that was most prominent corresponded to β-d-Galp-(1 → 6)-β-d-Galp-(1 → 4)-d-Glc (6′Gallac) and the second peak with β-d-Galp-(1 → 3)-β-d-Galp-(1 → 4)-d-Glc (3′Gallac). There was also evidence for β(1 → 6)-Gal elongation of allolactose, as well as β(1 → 6)- and β(1 → 3)-Gal elongations of the major disaccharide structures. The lower intensity DP4 fraction showed mainly structures with β(1 → 3)- or β(1 → 6)-Gal elongations of lactose or combinations of both types of linkages.
Graphical representation of structures identified in A GOS and B GlcOS; synthesized by rTmBglA from lactose and cellobiose, respectively. Structure numbers match with peaks in Figs. 1 and 6. The structure numbers of GOS used in this figure are the same as in previous publications (van Leeuwen et al. 2016; Yin et al. 2017b, 2018; Kittibunchakul et al. 2020)
GlcOS characterization
When incubated with cellobiose, the rTmBglA enzyme synthesized various GlcOS structures. The MALDI-TOF–MS spectrum (Fig. 3B) showed peaks at 365, 527, and 689 m/z indicating sodium-adduct peaks of Hex2, Hex3, and Hex4 structures, respectively. Besides glucose and cellobiose, three major peaks and several minor peaks were observed in the HPAEC-PAD profile (Fig. 6).
HPAEC-PAD profile of GlcOS synthesized by rTmBglA from 20%wt cellobiose (C2). Structures are identified based on reference standards, C3: cellotriose [β-(1 → 4)], C4: cellotetraose [β-(1 → 4)], G2: gentiobiose [β-(1 → 6)], L2: laminaribiose [β-(1 → 3)] and L3: laminaritriose [β-(1 → 3)], matching with identified structures shown in Fig. 5
The major structures formed are β-d-Glcp-(1 → 6)-d-Glc (gentiobiose; G2), β-d-Glcp-(1 → 3)-d-Glc (laminaribiose; L2), and β-d-Glcp-(1 → 4)-β-d-Glcp-(1 → 4)-d-Glc (cellotriose; C3). Minor amounts of laminaritriose (L3) and cellotetraose (C4) were also observed. A medium-sized peak was found at retention time 32.6 min between the elution times for C3 and C4. Gentiotriose (G3) and gentiotetraose (G4) standards were not available, but are expected to elute slightly before C3 and C4, respectively, based on the relative elution positions of G2 and C2. Possibly, G3 elutes at the same retention time as L2, for which a standard was available, and the peak at 32.6 min might be G4. Comparing the relative intensities of the G2 and C2 peaks, and the C3 and C4 peaks, it is also possible that the peak at 32.6 min is a β(1 → 3) elongation of C2 or a β(1 → 6) elongation of L2 or C3. Other minor peaks were not identified, from MALDI-TOF–MS analysis; however, only di- up to tetra-hexose structures were observed. The elution positions of these peaks lie in between cello-oligosaccharide and laminari-oligosaccharide standards, indicating tri- and tetrasaccharides with combinations of β(1 → 3), β(1 → 4), and β(1 → 6)-linkages. These cellobiose-derived trans-glucosylation products show a similar linkage preference for β(1 → 3) and β(1 → 6) linkages, as was observed for trans-galactosylation with lactose.
Discussion
The incubation with the commercial TmBglA preparation showed trans-glycosylation activity (Fig. 1). Initial optimization experiments showed that despite high thermostability, there is a limit to the gain of higher enzyme activity. When incubated at 90 °C, the enzyme showed clear activity, but the product showed significant browning, most likely a result of Maillard reaction. A higher dose of enzyme also increased browning, fitting the Maillard hypothesis. Incubations with 75%wt lactose at 60 °C showed difficulty in keeping lactose dissolved, as evidenced by large amounts of precipitate in the product (Supplementary Fig. S2). After 24-h incubation, the slurry still produced GOS; higher doses of enzyme resulted in less precipitated material in the final product. HPAEC-PAD analysis (not shown) of all the investigated incubation conditions clearly showed reduced lactose consumption ratios when 75%wt lactose was incubated at 60 °C. This indicates that a slurry or syrup can still be used efficiently in GOS synthesis, but high amounts of precipitated lactose limit the reaction.
The SDS-PAGE analysis of the commercial TmBglA preparation revealed multiple bands, indicating impurities. Since it cannot be excluded that an impurity is responsible for the activity towards lactose, the recombinant gene was ordered in a plasmid (GeneArt) and used to express rTmBglA, which was successfully purified (Supplementary Fig. S1). The recombinant enzyme appeared with a higher MW ~ 56 kDa on the SDS-PAGE gel, which is most likely the effect of the His6-tag, which has been described to disproportionately affect mobility in the gel (Niu and Guiltinan 1994). Comparison of GOS synthesized from lactose with the Megazyme TmBglA preparation and the purified rTmBglA enzyme showed the same product profiles (Supplementary Fig. S4). The trans-glycosylase activity with lactose thus is not based on a contaminant protein in the Megazyme TmBglA preparation, but is an inherent activity of the TmBglA enzyme itself. This observation also fits previous reports showing activity of this TmBglA towards lactose (Gabelsberger et al. 1993) and trans-glycosylase activity of a similar enzyme from T. naphthophila (Yang et al. 2018). The latter reports only production of 3′-galactosyllactose as a single product. Neither study has evaluated oligosaccharide synthesis with cellobiose as substrate.
Enzyme activity and kinetics
The kinetics of the enzyme with lactose showed a biphasic Lineweaver–Burk (Supplementary Fig. S3; Table 2). One set of kinetic parameters is for low-substrate concentrations, and one set is for high-substrate concentrations. Such biphasic plots also were observed for other GOS-synthesizing enzymes, e.g., for the β-galactosidase from B. circulans BgaD (Song et al. 2011b, 2011a; Bultema et al. 2014). Possibly, at higher substrate concentrations, the products of the trans-glycosylation reaction significantly influence the rate of Glc release from lactose. For cellobiose, such biphasic kinetics was not observed. This may indicate a more limited trans-glycosylation activity towards cellobiose. It is apparent that the catalytic rate with cellobiose, based on release of Glc, is significantly higher than with lactose. One early study showed that the specific activity with cellobiose is higher than with lactose (Gabelsberger et al. 1993). Gabelsberger et al. compared the reactions with cellobiose and lactose, showing 42 and 28%, respectively, of the activity with pNP-β-d-Glc (100%) (Gabelsberger et al. 1993). Gabelsberger et al. determined relative activities using 40 mM lactose and cellobiose at pH 6.2 and 75 °C. At low-substrate concentrations, the catalytic efficiency kcat/Km of rTmBglA is higher for lactose than for cellobiose, while at higher concentrations of lactose, the enzyme is less efficient than at low lactose concentrations. Further reactions of the enzyme with trans-glycosylation products as donor already in the early reaction phase do not result in release of Glc and were therefore not detected in both the reaction with lactose as well as with cellobiose. This may result in an underestimated catalytic rate and thereby an underestimated catalytic efficiency of the enzyme. It should be noted that the Km values with both cellobiose, as well as lactose, are relatively high, indicating low-substrate affinity. Possibly, this results in lower activity towards the remaining substrate near the end of the reaction when the substrates near depletion.
Yields were determined by quantitative HPAEC-PAD analysis of Glc and Gal, as well as residual lactose (Table 1; chromatograms not shown). Using rTmBglA enzyme, we obtained a maximal yield of 72.1%wt GOS from 570 g/L lactose, with only 10.5%wt remaining lactose. To the best of our knowledge, this is the highest yield obtained so far in enzymatic GOS production. The GOS yield observed for the commercially used B. circulans BgaD is 63.3%wt GOS with 21.2%wt remaining lactose after 22 h (Benjamins et al. 2014). Between 24 and 48 h, the GOS yield declined again, probably due to hydrolytic activity of the enzyme towards the GOS products. When the enzyme concentration was increased to 10 U/g lactose, a maximum GOS yield of 63.3%wt was obtained in 6-h reaction time (Table 1). The reduced yield at higher enzyme concentration is likely the effect of limited lactose availability per enzyme molecule, resulting in a relatively higher hydrolysis ratio. Previous work using immobilized T. maritima BglA showed a GOS yield of ~ 24%wt with free enzyme and 28%wt with immobilized enzyme (Alnadari et al. 2021). The latter study, however, used 200 g/L lactose and a relatively high enzyme load (270 U/mL) and screened for products by TLC analysis. These conditions are likely more conducive to hydrolysis than trans-glycosylation, which negatively influences GOS yields.
The yield efficiency of GOS in g/L is higher for the incubation with rTmBglA β-galactosidase activity at 3.75 U/g lactose, while the GOS yield in g/L/h is 3.5 times higher with rTmBglA at 10 U/g lactose (Table 1). From the Vmax with lactose (Table 2), the yield in g GOS per g enzyme can be calculated, showing that the incubation with 3.75 U/g lactose is much more efficient in terms of enzyme use. When the cost of the enzyme is the most important economic cost factor, GOS synthesis at 3.75 U/g lactose is most favorable. However, if bioreactor availability or production times are a limiting factor, it may be more economical to shorten reaction times with higher enzyme doses to increase the total product versus time ratio. Using similar enzyme-to-substrate conditions, from cellobiose, a 57.7%wt maximum yield was achieved. This is a promising relative yield, but relatively low on absolute conversion with 115 g/L and only 5.2 g/L/h conversion. Enzyme efficiency was calculated from the total yields and amount of enzyme added. In terms of enzyme efficiency, a yield of 3394 g GlcOS/mg enzyme was still ~ 80% compared with GOS yields at 4242 g GOS/mg enzyme (Table 1).
The rTmBglA GOS product mixture showed 20 GOS structures with a mixture of β(1 → 3), β(1 → 4), and β(1 → 6)-linkages, as well as remaining lactose, and free Gal and Glc. Previous studies have shown commercial GOS products with a focus towards β(1 → 3) and β(1 → 6), towards β(1 → 4) and β(1 → 6), or towards a single linkage preference. In case of commercial GOS preparations, some are the result of a combination of two enzymes. Previously, the β-galactosidase BgaD from B. circulans was adapted by a single-point mutation to produce GOS with a combined β(1 → 3) and β(1 → 4) linkage preference (Yin et al. 2017b). That study was the first example of a product with that combination of structures, culminating in structure 39b, which was observed there for the first time (Yin et al. 2017b). Our results show that rTmBglA is a naturally occurring enzyme capable of synthesizing β(1 → 3) and β(1 → 6) linked GOS from lactose, with also some β(1 → 4) epitopes. This combination of three types of GOS elongation, β(1 → 3), β(1 → 4), and β(1 → 6), has not been observed previously, rendering rTmBglA a very interesting example of a GOS-synthesizing enzyme.
When incubated with cellobiose, the product spectrum is less complex, showing mainly di- and trisaccharides. The maximum concentration of cellobiose that could be included in a solution or slurry (~ 20%wt) is significantly lower than for lactose (~ 65%wt) at 75 °C, limiting the cellobiose availability. Despite this limitation, a good conversion to cellobiose-derived di-, tri-, and tetrasaccharides was achieved, with mainly β(1 → 3) and β(1 → 6) elongations, similar to the GOS product with lactose as substrate. The relatively high concentrations of DP2 products gentiobiose and laminaribiose suggest that initially released free Glc is a good acceptor molecule. Just as with lactose as substrate, the cellobiose incubation also shows some β(1 → 4) elongations, such as the formation of cellotriose and cellotetraose.
This paper shows that the β-glucosidase BglA from T. maritima is a versatile, thermostable enzyme capable of synthesizing a variety of different oligosaccharide mixtures at higher yields than observed so far. Based on previous literature, the GOS mixture from lactose, containing mainly β(1 → 3) and β(1 → 6)-linked Gal residues, are very promising bifidogenic products.
Data availability
Data will be made available on reasonable request.
References
Alizadeh A, Akbari P, Difilippo E, Schols HA, Ulfman LH, Schoterman MHC, Garssen J, Fink-Gremmels J, Braber S (2016) The piglet as a model for studying dietary components in infant diets: effects of galacto-oligosaccharides on intestinal functions. Brit J Nutr 115:605–618. https://doi.org/10.1017/S0007114515004997
Alnadari F, Xue Y, Almakas A, Mohedein A, Samie A, Abdel‐Shafi M, Abdin M (2021) Large batch production of galactooligosaccharides using β‐glucosidase immobilized on chitosan‐functionalized magnetic nanoparticle. J Food Biochem 45. https://doi.org/10.1111/jfbc.13589
Arreola SL, Intanon M, Suljic J, Kittl R, Pham NH, Kosma P, Haltrich D, Nguyen TH (2014) Two β-galactosidases from the human isolate Bifidobacterium breve DSM 20213: Molecular cloning and expression, biochemical characterization and synthesis of galacto-oligosaccharides. PLoS One 9. https://doi.org/10.1371/journal.pone.0104056
Benjamins E, Boxem L, KleinJan-Noeverman J, Broekhuis TA (2014) Assessment of repetitive batch-wise synthesis of galacto-oligosaccharides from lactose slurry using immobilised β-galactosidase from Bacillus circulans. Int Dairy J 38:160–168. https://doi.org/10.1016/j.idairyj.2014.03.011
Bindels LB, Delzenne NM, Cani PD, Walter J (2015) Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 12:303–310. https://doi.org/10.1038/nrgastro.2015.47
Bultema JB, Kuipers BJH, Dijkhuizen L (2014) Biochemical characterization of mutants in the active site residues of the β-galactosidase enzyme of Bacillus circulans ATCC 31382. FEBS Open Bio 4:1015–1020. https://doi.org/10.1016/j.fob.2014.11.002
Cardelle-Cobas A, Corzo N, Olano A, Peláez C, Requena T, Ávila M (2011) Galactooligosaccharides derived from lactose and lactulose: influence of structure on Lactobacillus, Streptococcus and Bifidobacterium growth. Int J Food Microbiol 149:81–87. https://doi.org/10.1016/j.ijfoodmicro.2011.05.026
Cardelle-Cobas A, Villamiel M, Olano A, Corzo N (2008) Study of galacto-oligosaccharide formation from lactose using Pectinex Ultra SP-L. J Sci Food Agric 88:954–961. https://doi.org/10.1002/jsfa.3173
Davis LMG, Martínez I, Walter J, Hutkins R (2010) A dose dependent impact of prebiotic galactooligosaccharides on the intestinal microbiota of healthy adults. Int J Food Microbiol 144:285–292. https://doi.org/10.1016/j.ijfoodmicro.2010.10.007
Drula E, Garron M-L, Dogan S, Lombard V, Henrissat B, Terrapon N (2022) The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res 50:D571–D577. https://doi.org/10.1093/nar/gkab1045
Ferreira-Lazarte A, Olano A, Villamiel M, Moreno FJ (2017) Assessment of in vitro digestibility of dietary carbohydrates using rat small intestinal extract. J Agric Food Chem 65:8046–8053. https://doi.org/10.1021/acs.jafc.7b01809
Füreder V, Rodriguez-Colinas B, Cervantes FV, Fernandez-Arrojo L, Poveda A, Jimenez-Barbero J, Ballesteros AO, Plou FJ (2020) Selective synthesis of galactooligosaccharides containing β(1→3) linkages with β-galactosidase from Bifidobacterium bifidum (Saphera). J Agric Food Chem 68:4930–4938. https://doi.org/10.1021/acs.jafc.0c00997
Gabelsberger J, Liebl W, Schleifer K-H (1993) Purification and properties of recombinant ?-glucosidase of the hyperthermophilic bacterium Thermotoga maritima. Appl Microbiol Biotechnol 40. https://doi.org/10.1007/BF00170427
Goulas T, Goulas A, Tzortzis G, Gibson GR (2009) Comparative analysis of four β-galactosidases from Bifidobacterium bifidum NCIMB41171: purification and biochemical characterisation. Appl Microbiol Biotechnol 82:1079–1088. https://doi.org/10.1007/s00253-008-1795-5
Goyal K, Selvakumar P, Hayashi K (2001) Characterization of a thermostable beta-glucosidase (BglB) from Thermotoga maritima showing transglycosylation activity. J Mol Catal B-Enzym 15:45–53
Jung SJ, Houde R, Baurhoo B, Zhao X, Lee BH (2008) Effects of galacto-oligosaccharides and a Bifidobacteria lactis-based probiotic strain on the growth performance and fecal microflora of broiler chickens. Poult Sci 87:1694–1699. https://doi.org/10.3382/ps.2007-00489
Kittibunchakul S, van Leeuwen SS, Dijkhuizen L, Haltrich D, Nguyen T-H (2020) Structural comparison of different galacto-oligosaccharide mixtures formed by β-galactosidases from lactic acid bacteria and bifidobacteria. J Agric Food Chem 68:4437–4446. https://doi.org/10.1021/acs.jafc.9b08156
Kothari D, Goyal A (2015) Gentio-oligosaccharides from Leuconostoc mesenteroides NRRL B-1426 dextransucrase as prebiotics and as a supplement for functional foods with anti-cancer properties. Food Funct 6:604–611. https://doi.org/10.1039/C4FO00802B
Lee JM, Jang WJ, Lee E-W, Kong I-S (2020) β-Glucooligosaccharides derived from barley β-glucan promote growth of lactic acid bacteria and enhance nisin Z secretion by Lactococcus lactis. LWT 122:109014. https://doi.org/10.1016/j.lwt.2020.109014
Nguyen T-H, Splechtna B, Steinböck M, Kneifel W, Lettner HP, Kulbe KD, Haltrich D (2006) Purification and characterization of two novel β-galactosidases from Lactobacillus reuteri. J Agric Food Chem 54:4989–4998. https://doi.org/10.1021/jf053126u
Niu X, Guiltinan MJ (1994) DNA binding specificity of the wheat bZIP protein EmBP-1. Nucleic Acids Res 22:4969–4978. https://doi.org/10.1093/nar/22.23.4969
Oozeer R, van Limpt K, Ludwig T, Ben Amor K, Martin R, Wind RD, Boehm G, Knol J (2013) Intestinal microbiology in early life: specific prebiotics can have similar functionalities as human-milk oligosaccharides. Am J Clin Nutr 98:561S-571S. https://doi.org/10.3945/ajcn.112.038893
Rodriguez-Colinas B, De Abreu MA, Fernandez-Arrojo L, De Beer R, Poveda A, Jimenez-Barbero J, Haltrich D, Ballesteros Olmo AO, Fernandez-Lobato M, Plou FJ (2011) Production of galacto-oligosaccharides by the β-galactosidase from Kluyveromyces lactis: comparative analysis of permeabilized cells versus soluble enzyme. J Agric Food Chem 59:10477–10484. https://doi.org/10.1021/jf2022012
Rycroft CE, Jones MR, Gibson GR, Rastall RA (2001) Fermentation properties of gentio-oligosaccharides. Lett Appl Microbiol 32:156–161. https://doi.org/10.1046/j.1472-765x.2001.00875.x
Sahasrabudhe NM, Tian L, van den Berg M, Bruggeman G, Bruininx E, Schols HA, Faas MM, de Vos P (2016) Endo-glucanase digestion of oat β-glucan enhances Dectin-1 activation in human dendritic cells. J Funct Foods 21:104–112. https://doi.org/10.1016/j.jff.2015.11.037
Song J, Abe K, Imanaka H, Imamura K, Minoda M, Yamaguchi S, Nakanishi K (2011a) Causes of the production of multiple forms of β-galactosidase by Bacillus circulans. Biosci Biotechnol Biochem 75:268–278. https://doi.org/10.1271/bbb.100574
Song J, Imanaka H, Imamura K, Minoda M, Katase T, Hoshi Y, Yamaguchi S, Nakanishi K (2011b) Cloning and expression of a β-galactosidase gene of Bacillus circulans. Biosci Biotechnol Biochem 75:1194–1197. https://doi.org/10.1271/bbb.110014
Van Leeuwen SS, Kuipers BJH, Dijkhuizen L, Kamerling JP (2014) 1H NMR analysis of the lactose/β-galactosidase-derived galacto-oligosaccharide components of Vivinal® GOS up to DP5. Carbohydr Res 400:59–73. https://doi.org/10.1016/j.carres.2014.08.012
van Leeuwen SS, Kuipers BJHH, Dijkhuizen L, Kamerling JP, Van Leeuwen SS, Kuipers BJHH, Dijkhuizen L, Kamerling JP (2016) Comparative structural characterization of 7 commercial galacto-oligosaccharide (GOS) products. Carbohydr Res 425:48–58. https://doi.org/10.1016/j.carres.2016.03.006
Vulevic J, Juric A, Walton GE, Claus SP, Tzortzis G, Toward RE, Gibson GR (2015) Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Brit J Nutr 114:586–595. https://doi.org/10.1017/S0007114515001889
Yan YL, Hu Y, Gänzle MG (2018) Prebiotics, FODMAPs and dietary fiber — conflicting concepts in development of functional food products? Curr Opin Food Sci 20:30–37. https://doi.org/10.1016/j.cofs.2018.02.009
Yang J, Gao R, Zhou Y, Anankanbil S, Li J, Xie G, Guo Z (2018) β-glucosidase from Thermotoga naphthophila RKU-10 for exclusive synthesis of galactotrisaccharides: Kinetics and thermodynamics insight into reaction mechanism. Food Chem 240:422–429. https://doi.org/10.1016/j.foodchem.2017.07.155
Yin H, Bultema JB, Dijkhuizen L, van Leeuwen SS (2017a) Reaction kinetics and galactooligosaccharide product profiles of the β-galactosidases from Bacillus circulans, Kluyveromyces lactis and Aspergillus oryzae. Food Chem 225:230–238. https://doi.org/10.1016/j.foodchem.2017.01.030
Yin H, Dijkhuizen L, van Leeuwen SS (2018) Synthesis of galacto-oligosaccharides derived from lactulose by wild-type and mutant β-galactosidase enzymes from Bacillus circulans ATCC 31382. Carbohydr Res 465:58–65. https://doi.org/10.1016/j.carres.2018.06.009
Yin H, Pijning T, Meng X, Dijkhuizen L, Van Leeuwen SS (2017b) Engineering of the Bacillus circulans β-galactosidase product specificity. Biochemistry 56:704–711. https://doi.org/10.1021/acs.biochem.7b00032
Yu L, Gao Y, Ye Z, Duan H, Zhao J, Zhang H, Narbad A, Tian F, Zhai Q, Chen W (2023) Interaction of beta-glucans with gut microbiota: dietary origins, structures, degradation, metabolism, and beneficial function. Crit Rev Food Sci Nutr 1–26. https://doi.org/10.1080/10408398.2023.2217727
Funding
The study was fully funded by University of Groningen and has received no external funding.
Author information
Authors and Affiliations
Contributions
GAtK: conducted experiments, curated data, and manuscript preparation; PS: conception of research, data review and editing manuscript; LD: supervision, data review, and editing manuscript; SSvL: conception and supervision of research, data review, manuscript preparation, and revision. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
Geralt A. ten Kate is currently employed by FrieslandCampina. The company had no participation in the study or any influence on the contents of this paper.
Peter Sanders declares no conflict of interest.
Sander S. van Leeuwen declares no conflict of interest.
Lubbert Dijkhuizen is currently employed at Carbexplore Research BV, a company that produces and markets β-galactosidase enzymes and their reaction products. The company had no participation in the study or any influence on the contents of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
ten Kate, G.A., Sanders, P., Dijkhuizen, L. et al. Kinetics and products of Thermotoga maritima β-glucosidase with lactose and cellobiose. Appl Microbiol Biotechnol 108, 349 (2024). https://doi.org/10.1007/s00253-024-13183-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13183-6