Abstract
Ethylene glycol (EG) is an industrially important two-carbon diol used as a solvent, antifreeze agent, and building block of polymers such as poly(ethylene terephthalate) (PET). Recently, the use of EG as a starting material for the production of bio-fuels or bio-chemicals is gaining attention as a sustainable process since EG can be derived from materials not competing with human food stocks including CO2, syngas, lignocellulolytic biomass, and PET waste. In order to design and construct microbial process for the conversion of EG to value-added chemicals, microbes capable of catabolizing EG such as Escherichia coli, Pseudomonas putida, Rhodococcus jostii, Ideonella sakaiensis, Paracoccus denitrificans, and Acetobacterium woodii are candidates of chassis for the construction of synthetic pathways. In this mini-review, we describe EG catabolic pathways and catabolic enzymes in these microbes, and further review recent advances in microbial conversion of EG to value-added chemicals by means of metabolic engineering.
Key points
• Ethylene glycol is a potential next-generation feedstock for sustainable industry.
• Microbial conversion of ethylene glycol to value-added chemicals is gaining attention.
• Ethylene glycol-utilizing microbes are useful as chassis for synthetic pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
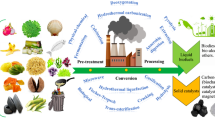
Ethylene glycol (EG) is an industrially important two-carbon diol used as a solvent, antifreeze agent, and building block of polymers such as poly(ethylene terephthalate) (PET). Currently, EG is predominantly produced from fossil fuels by hydration of ethylene oxide, while a small amount of EG is produced from renewable resources by dehydration of biobased ethanol. In addition, microbial production of EG from plant-derived sugars, i.e., d-glucose and d-xylose, has been extensively studied by several groups (Salusjärvi et al. 2019). Recent development of electrochemical and chemical processes enabled selective production of EG from CO2 via ethylene (Tamura et al. 2015; Lum et al. 2020; Leow et al. 2020; Fan et al. 2023). These technological advances expanded the potential role of EG in addition to traditional applications; EG is now becoming a promising feedstock that can be obtained from CO2. Furthermore, EG also can be obtained from syngas (Satapathy et al. 2018; Sun and Chai 2022), lignocellulolytic biomass (Pang et al. 2011; Li et al. 2012; te Molder et al. 2021), and PET wastes (Werner et al. 2021; Diao et al. 2023); all of them do not compete with human food stocks (Fig. 1). In this context, microbial conversion of EG to value-added chemicals is gaining attention as a sustainable process (Wagner et al. 2023b) (Fig. 1). In order to design and construct microbial cell factories with EG as the starting material, microbes capable of assimilating EG are useful as chassis for synthetic pathways converting EG to value-added chemicals.
EG assimilation has been reported in diverse microbes including Clostridium glycolicum (Gaston and Stadtman 1963), Flavobacterium sp. (Child and Willetts 1978), Halomonas elongate (Gonzalez et al. 1972), Escherichia coli (Boronat et al. 1983), Pseudomonas putida (Mückschel et al. 2012), Acetobacterium woodii (Trifunović et al. 2016), Ideonella sakaiensis (Hachisuka et al. 2022), Rhodococcus jostii (Shimizu et al. 2024), and Paracoccus denitrificans (Bordel et al. 2024). Microbial EG catabolism exhibits diversity in routes and enzymes involved in Fig. 2. The aerobic EG catabolism proceeds via a common sequential oxidation of EG to glyoxylate, where enzymes and cofactors involved in the initial oxidation of EG are varied among microbes (Fig. 2a). Subsequently, the resulting glyoxylate is further assimilated via the glycerate pathway (Fig. 2b) or the β-hydroxyaspartate cycle (BHAC) (Fig. 2c). In the anaerobic EG catabolism found in acetogens such as A. woodie, EG is dehydrated to acetaldehyde by diol dehydratase (Fig. 2d). In this mini-review, we describe EG catabolic pathways and catabolic enzymes in several microbes, and further review their applications for microbial conversion of EG to value-added chemicals including strain improvement by metabolic engineering.
FucO-mediated ethylene glycol catabolism in Escherichia coli
E. coli is a gram-negative model bacterium providing a number of genetic tools for design and strain construction for the bioproduction of a variety of chemicals from renewable resources. Although the wild-type E. coli is unable to use EG, spontaneous mutants obtained from l-1,2-propanediol utilizing mutants were able to grow with EG as a sole source of carbon and energy (Boronat et al. 1983). It has been proposed that constitutive expression of fucO encoding 1,2-propandiol oxidoreductase and aldA encoding glycolaldehyde dehydrogenase is essential for EG catabolism by E. coli, where FucO catalyzes NAD+-dependent oxidation of EG to glycolaldehyde (Boronat and Aguilar 1979), and AldA catalyzes NAD(P)+-dependent oxidation of glycolaldehyde to glycolate (Caballero et al. 1983) (Fig. 2a). It should be noted that a recent study revealed that expression of fucO and deletion of yqhD encoding highly active aldehyde reductase is sufficient for EG assimilation by E. coli (Frazão et al. 2023). After the reactions catalyzed by FucO and AldA, the resulting glycolate is oxidized by glycolate dehydrogenase (GlcDEF) to glyoxylate (Pellicer et al. 1996), and further catabolized to 2-phospho-d-glycerate, an intermediate of glycolysis, via the glycerate pathway (Fig. 2b) including glyoxylate carboligase, (Gcl) (Chang et al. 1993), tartronate semialdehyde reductase (GlxR) (Njau et al. 2000), and d-glycerate 2-kinase (GlxK) (Rodionova et al. 2023). Note that GlxK was initially reported to phosphorylate the C3-position of d-glycerate (Doughty et al. 1966), whereas subsequent studies revealed that GlxK actually phosphorylates the C2-position of d-glycerate (Bartsch et al. 2008; Zelcbuch et al. 2015).
FucO, the key enzyme of EG catabolism in E. coli, is a member of group III alcohol dehydrogenase, which contains Fe(II) atom at the catalytic center (Montella et al. 2005; Zavarise et al. 2023). FucO is inactivated under aerobic conditions and physiologically catalyzes the reduction of l-lactaldehyde to l-1,2-propanediol for oxidation of NADH during anaerobic l-fucose and l-rhamnose utilization (Chen et al. 1987). The oxygen sensitivity of FucO is important to prevent the formation of l-1,2-propanediol under aerobic conditions (Baldomà and Aguilar 1988), whereas I6L/L7V mutant of FucO is known to be oxygen-resistant (Lu et al. 1998).
Microbial conversion of EG to value-added chemicals by genetically engineered E. coli has been reported by several groups for the production of multiple target compounds (Table 1). Pandit and co-workers reported glycolate production from EG using E. coli with overexpression of oxygen-tolerant fucO and aldA (Pandit et al. 2021). Using the orthogonal matrix approach, they evaluated several feedstocks including glucose, xylose, formate, and EG as substrates for glycolate production and found that EG is the best substrate for glycolate production in E. coli. Subsequent flux-balance analysis and flux variability analysis revealed that the oxygen supply is important to control glycolate production by E. coli. Finally, the engineered E. coli strain produced a maximum titer of 10.4 g/L glycolate from EG under optimized conditions (Pandit et al. 2021). In this context, some yeast and acetic acid bacteria are also known to produce glycolate from EG without assimilation (Kataoka et al. 2001; Wei et al. 2009). The best example of glycolate production from EG is the cell reaction of the acetic acid bacterium Gluconobacter oxydans, which produced 63.3 g/L glycolate at a yield of 97.2% under optimized conditions (Hua et al. 2018).
Panda and co-workers recently reported the conversion of EG to aromatic compounds including l-tyrosine, l-phenylalanine, and p-coumarate using genetically engineered E. coli (Table 1) with plasmids expressing oxygen-tolerant fucO, aldA, feedback-resistant tyrA, and feedback-resistant aroG (Panda et al. 2023) (Fig. 3). The best strain produced 2 g/L l-tyrosine from 10 g/L EG, which corresponds to almost 50% of theoretical yield. A similar titer was achieved when acid hydrolysates of PET waste were used as the starting material.
Microbial conversion of ethylene glycol to l-tyrosine by genetically engineered E. coli. Overexpressed genes are shown in blue. FucOI6L/L7V, I6L/L7V mutant of l-1,2-propandiol oxidoreductase; AldA, l-lactaldehyde dehydrogenase; AroG, DAHP synthase; TyrA, chorismate mutase/prephenate dehydrogenase; DAHP, 3-deoxy-d-arabino-heptulosonate 7-phosphate; 4HPP, 4-hydroxyphenylpyruvate
Frazao and co-workers achieved the microbial production of 2,4-dihydroxybutyric acid (DHB), a non-natural precursor for a methionine analogue or 1,3-propanediol, from EG using E. coli implemented with a synthetic pathway (Frazão et al. 2023) (Fig. 4). They screened enzymes for the synthetic pathway for the conversion of glycolaldehyde to DHB. After establishment of an E. coli strain producing DHB from glycolaldehyde, the pathway was extended for EG utilization by introducing EG-oxidizing enzymes (Fig. 4). Notably, the best DHB titer from EG, 0.8 g/L (Table 1), was achieved by the expression of Gox0313 (Zhang et al. 2015), NAD+-dependent alcohol dehydrogenase from G. oxydans, not endogenous fucO nor its oxygen-tolerant mutant.
Microbial conversion of ethylene glycol to 2,4-dihydroxybutyrate by genetically engineered E. coli. GO.Adh, NAD+-dependent alcohol dehydrogenase Gox0313 from G. oxydans; Ec.FsaAL107Y/A129G, L107Y/A129G mutant of d-fructose 6-phosphate aldolase from E. coli; Pc.TadH, d-threo-aldose 1-dehydrogenase from Paraburkholderia caryophylli; Tt.Lac11, gluconolactonase from Thermogutta terrifontis; Hh.AraD, d-arabinonate dehydratase from Herbaspirillum huttiense; Ec.Mdh5Q, I12V/R81A/M85Q/D86S/G179D mutant of l-malate dehydrogenase from E. coli
PedEH-mediated ethylene glycol catabolism in Pseudomonas putida
P. putida is a gram-negative metabolically versatile bacterium that has been used for various biotechnological applications (Salusjärvi et al. 2019). Mückschel and co-workers examined the utilization of EG by two P. putida strains, JM37 and KT2440, and they found that P. putida JM37 was able to grow with EG as a sole source of carbon and energy, whereas P. putida KT2440 was unable to grow with EG (Mückschel et al. 2012). Proteome analysis revealed that PQQ-dependent alcohol dehydrogenases (PedEH), aldehyde dehydrogenase (PedI), and isocitrate lyase (AceA) were upregulated in P. putida KT2440 upon EG treatment, whereas Gcl was not (Mückschel et al. 2012). Subsequent adaptive laboratory evolution experiments revealed that the activation of the glycerate pathway including glyoxylate carboligase (Gcl), hydroxypyruvate isomerase (Hyi), tartronate semialdehyde reductase (GlxR), d-glycerate 2-kinase (TtuD), and pyruvate kinase (PyrK) by deletion of gclR encoding a GntR family transcriptional regulator enabled P. putida KT2440 to grow with EG as a sole source of carbon and energy (Li et al. 2019).
A major difference between EG assimilation in E. coli and that in P. putida is enzymes involved in the oxidation of EG; FucO in E. coli uses NAD+ for the electron acceptor, whereas both PedE and PedH in P. putida use PQQ for the electron acceptor. As shown in Fig. 5, the chemical structures of NAD+ and PQQ are completely different. Unlike the ubiquitous molecule NAD+ found in all domains of life, PQQ is only found in some prokaryotes including methylotrophs (Keltjens et al. 2014), and that at least six genes are required for PQQ biosynthesis (Puehringer et al. 2008). A unique feature of PQQ is its high midpoint potential (90 mV), the value of which is significantly higher than that of NAD+ (− 340 mV). Notably, the high midpoint potential of PQQ is advantageous to drive EG oxidation by PedEH, as compared to FucO that rather prefers NADH-dependent reduction of glycolaldehyde at neutral pH (Boronat and Aguilar 1979).
Biotechnological applications of P. putida strains for microbial conversion of EG or its derivatives to value-added chemicals have been reported by several groups (Table 1). The earlier work is poly(hydroxyalkanoate) (PHA) production from EG reported by Franden and co-workers (Franden et al. 2018). They firstly analyzed the minimum genes in the gcl operon required for EG assimilation by P. putida. Among genes in the gcl operon (Fig. 6), only two genes, gcl and glxR, were required for growth with EG, whereas expression of the entire gcl operon further improved growth with EG. It was also revealed that overexpression of glcDEF along with overexpression of the glycerate pathway improved EG utilization by P. putida. The resulting strain MFL185 was able to consume 500 mM EG within 120 h, and produced 32.19% dry cell weight of C8-C14 PHA from EG (Table 1).
Werner and co-workers achieved β-ketoadipic acid (βKA) production from bis(2-hydroxyethyl)terephthalate (BHET), 1.5-mer of PET, or chemically depolymerized PET using genetically engineered P. putida (Werner et al. 2021). Based on EG-assimilating strain ΔgclR, deletion of pcaIJ involved in βKA assimilation (Parales and Harwood 1992, 1993) and introduction of terephthalate (TPA) catabolic genes (tphA2IIA3IIBIIA1II) from Comamonas sp. E6 (Sasoh et al. 2006), TPA transporter (tpaK) from R. jostii RHA1 (Hara et al. 2007), and PET-degrading genes from I. sakaiensis (Yoshida et al. 2016) resulted in direct conversion of BHET or depolymerized PET to βKA (Fig. 7) with titers of 15.1 g/L and 0.22 g/L, respectively (Werner et al. 2021) (Table 1). Notably, accumulation of EG was observed during βKA production from BHET, suggesting that βKA inhibited EG utilization of P. putida by an unknown mechanism (Werner et al. 2021). In order to utilize both TPA and EG efficiently, Bao and co-workers used the consortium involving two P. putida strains, specializing in TPA and EG utilization, respectively (Bao et al. 2023). Comparing to the mono-culture approach, the consortium produced higher titer of PHA (0.64 g/L) and cis, cis-muconic acid (4.73 g/L) from PET hydrolysate, respectively (Table 1).
Chemoenzymatic synthesis of β-ketoadipate from poly(ethylene terephthalate). a Chemical depolymerization of poly(ethylene terephthalate) (PET) to bis(2-hydroxyethyl)terephthalate (BHET) catalyzed by titanium butoxide in ethylene glycol. b Microbial conversion of BHET to β-ketoadipate by genetically engineered P. putida. Overexpressed genes and deleted genes are shown in blue and red, respectively. PETaseIS, PET hydrolase from I. sakaiensis; MHETaseIS, mono(2-hydroxyethyl) terephthalate (MHET) hydrolase from I. sakaiensis; TpaKRHA1, terephthalate (TPA) transporter from R. jostii RHA1; TphAII E6, terephthalate 1,2-dioxygenase from Comamonas sp. E6; TphBII E6, 1,2-dihydroxy-3,5-cyclohexadiene-1,4-dicarboxylate (DCD) dehydrogenase from Comamonas sp. E6; GlcDEF, endogenous glycolate dehydrogenase
Tiso and co-workers investigated hydroxyalkanoyloxy-alkanoates (HAA) production from enzymatically depolymerized PET (Tiso et al. 2021) by using Pseudomonas umsongensis GO16 capable of assimilating TPA with endogenous TPA catabolic genes (Narancic et al. 2021). Since the strain GO16 was unable to utilize EG, they performed adaptive laboratory evolution. As a result, the obtained EG-assimilating mutant strain GO16 KS3 produced 35 mg/L HAA from enzymatically depolymerized PET (Table 1). Nevertheless, HAA production by the strain KS3 solely relied on TPA (Tiso et al. 2021).
EgaA-mediated ethylene glycol catabolism in Rhodococcus jostii
R. jostii is a gram-positive mycolic acid-containing bacterium known to be able to degrade various aromatic compounds including TPA (Hara et al. 2007). Due to its metabolic versatility and availability of tools for genome engineering (Liang and Yu 2021; Round et al. 2021), R. jostii has been used for several biotechnological applications (Donini et al. 2021). EG utilization by R. jostii was initially reported by Diao and co-workers in an attempt for upcycling of PET waste to lycopene by engineered strains derived from R. jostii PET (Diao et al. 2023); however, the EG catabolic pathway and EG catabolic enzymes in R. jostii PET were yet to be elucidated. Recently, we found that R. jostii strain RHA1 also can grow with EG as a source of carbon and energy (Shimizu et al. 2024). Biochemical and genetic analyses revealed that a mycofactocin (MFT)-associated dehydrogenase (EgaA) is responsible for the oxidation of EG to glycolaldehyde, and two aldehyde dehydrogenases (AldA1 and AldA2) might be involved in the oxidation of glycolaldehyde to glycolate, which is further catabolized by the glycerate pathway (Shimizu et al. 2024). It has been also reported that RHA1_ro02984 protein catalyzes consecutive oxidation of glycolaldehyde to oxalate during lignin oxidation by R. jostii RHA1 (Alruwaili et al. 2023), whereas the corresponding gene was not upregulated during growth with EG (Shimizu et al. 2024).
A unique feature of EG catabolism in R. jostii is the involvement of a MFT-associated dehydrogenase in the oxidation of EG (Fig. 2a). MFT is a group of ribosomally synthesized and posttranslationally modified peptides (Fig. 5) that has been suggested to act as a redox cofactor for the oxidation of various alcohols in Actinobacteria including the genus Rhodococcus (Ayikpoe et al. 2019). Indeed, the deletion of egaA negatively affected ethanol, 1-propanol, l-1,2-propanediol, and 1-butanol assimilation in addition to EG, suggesting that EgaA is responsible for the oxidation of various alcohols in R. jostii RHA1 (Shimizu et al. 2024). Although the midpoint potential of MFT is currently not known, that of premycofactocin, a precursor of MFT lacking the glycosyl-moiety, has been reported to be − 225 mV (Ayikpoe and Latham 2019).
As R. jostii is able to assimilate both EG and TPA constituting PET, this bacterium is a promising chassis for upcycling of PET to value-added chemicals. In this context, Diao and co-workers examined the upcycling of alkaline hydrolysates of PET to lycopene using genetically engineered R. jostii PET (Diao et al. 2023). The study revealed that the bacterium can use EG and TPA simultaneously, and achieved approximately 1300 µg/L lycopene from alkaline hydrolysates of PET by deletion of a putative lycopene β-cyclase gene (crtL-b) and optimization of the 2-methylerythritol 4-phosphate pathway for carotenoid biosynthesis (Diao et al. 2023).
Ethylene glycol catabolism in the PET assimilating bacterium Ideonella sakaiensis
I. sakaiensis is a gram-negative bacterium capable of assimilating PET as a sole source of carbon and energy (Yoshida et al. 2016). Since PET is a co-polymer consisting of EG and TPA, equivalent moles of EG and TPA are released during PET degradation catalyzed by PETase (Liu et al. 2023) and MHETase (Palm et al. 2019) produced by I. sakaiensis. Based on genome information, I. sakaiensis was predicted to be able to utilize TPA (Yoshida et al. 2016), and a recent study revealed that I. sakaiensis is able to grow with EG as a sole source of carbon and energy (Hachisuka et al. 2022). Genetic and biochemical analyses revealed that I. sakaiensis uses three PQQ-dependent alcohol dehydrogenases, PedE, PedH, and XoxF, for the oxidation of EG to glycolaldehyde and aldehyde dehydrogenase (PedI) for NAD+-dependent oxidation of glycolaldehyde to glycolate (Hachisuka et al. 2022) (Fig. 2a). Among three PQQ-dependent alcohol dehydrogenases, PedE exhibited Ca2+-dependent dehydrogenase activity towards various alcohols, whereas PedH and XoxF exhibited Pr3+-dependent dehydrogenase activities, where PedH preferred short-chain alcohols and XoxF preferred long-chain alcohols (Hachisuka et al. 2022). Based on genome information, I. sakaiensis was proposed to use the glycerate pathway for glyoxylate assimilation.
Since I. sakaiensis can utilize PET directly without chemical depolymerization, the application of this bacterium enables one-pot bioconversion of PET to value-added chemicals. The pioneering work by Fujiwara and co-workers is upcycling of PET to PHA using the wild-type I. sakaiensis (Fujiwara et al. 2021). They found PHA biosynthetic genes in the genome of I. sakaiensis, and confirmed PHA accumulation during growth with PET. After optimization of culture conditions, I. sakaiensis accumulated PHA from PET up to 48% of dry cell weight, which corresponds to PHA titer of 0.75 g/L (Table 1). In addition, a subsequent study revealed substrate specificity of PHA synthase from I. sakaiensis (Tan et al. 2022). Recently, a protocol for gene manipulation of I. sakaiensis was established (Hachisuka et al. 2021), enabling metabolic engineering of I. sakaiensis for the production of various chemicals from PET waste.
Ethylene glycol assimilation via the β-hydroxyaspartate cycle in Paracoccus denitrificans
P. denitrificans is a gram-negative bacterium that has been known to assimilate glyoxylate via the BHAC (Kornberg and Morris 1965). Recent study elucidated four enzymes constituting the BHAC (Schada von Borzyskowski et al. 2019): aspartate-glyoxylate aminotransferase (BhcA), β-hydroxyaspartate dehydratase (BhcB), β-hydroxyaspartate aldolase (BhcC), and iminosuccinate reductase (BhcD) (Fig. 2c). This study also revealed that the BHAC is distributed in a certain population of marine bacteria to use glycolate produced by marine algae and seaweeds via the oxygenase reaction of rubisco (Schada von Borzyskowski et al. 2019). Very recently, Bordel and co-workers found that P. denitrificans is able to grow with EG as a source of carbon and energy (Bordel et al. 2024). Based on its genome information, P. denitrificans is predicted to use FucO, AldA, and GlcDEF for sequential oxidation of EG to glyoxylate, which is further converted to oxaloacetate via the BHAC for biomass and energy (Bordel et al. 2024).
The BHAC is considered to be a more efficient carbon conserving C2-assimilating pathway than the glycerate pathway since the BHAC does not produce CO2 and does not require ATP for glyoxylate assimilation (Schada von Borzyskowski et al. 2019; Borzyskowski et al. 2020; Diehl et al. 2023) (Fig. 2c). Indeed, the implementation of the BHAC to the gcl deletion mutant of P. putida KT2440 and adaptive laboratory evolution resulted in both higher growth rates and biomass yields with EG as compared to the E6.1 strain that has been evolved to assimilate EG by the glycerate pathway (von Borzyskowski et al. 2023).
Anaerobic ethylene glycol catabolism in Acetobacterium woodii
The obligate anaerobic acetogenic bacterium A. woodie can grow with EG under strict anaerobic conditions with the formation of acetate and ethanol (Trifunović et al. 2016). Although aerobic EG catabolic pathways start with the oxidation of EG to form glycolaldehyde, the anaerobic EG catabolic pathway in A. woodii starts with the dehydration of EG to form acetaldehyde by propanediol dehydratase (PduCDE), which is known to be very oxygen-sensitive (Hartmanis and Stadtman 1986). The resulting acetaldehyde is further oxidized by CoA-dependent propionaldehyde dehydrogenase (PduP) to form acetyl-CoA with concomitant reduction of NAD+ to NADH, which is assumed to be re-oxidized to NAD+ through the reduction of acetaldehyde to ethanol by a yet-to-be identified alcohol dehydrogenase (Trifunović et al. 2016) (Fig. 2d). Notably, PduCDEP are also responsible for l-1,2-propanediol catabolism by A. woodii, which involves the formation of bacterial microcompartments presumably for the protection of cells from toxic aldehyde intermediates (Schuchmann et al. 2015).
In the purpose of bioconversion of EG to value-added chemicals, the use of acetogenic bacteria including A. woodii would be challenging since they obligately accumulate acetate and/or alcohols during growth to gain ATP and mediate cellular redox-state under anaerobic conditions (Katsyv and Müller 2020). However, it should be noted that acetogenic bacteria are capable of utilizing CO2 as the carbon source via the Wood-Ljungdahl pathway (Basen and Müller 2023), allowing direct bioconversion of CO2 to value-added chemicals including EG (Liew et al. 2022; Bourgade et al. 2022).
Conclusions and future prospects
As reviewed here, minimum requirements for the aerobic EG utilization are likely to be two metabolic modules: (i) oxidative conversion of EG to glyoxylate (three reactions) and (ii) glyoxylate assimilation via either the glycerate pathway (three reactions) or the BHAC (four reactions). Therefore, conferring the ability for EG utilization to non-EG-assimilating microbes would be time-consuming since enzymes for at least six reactions are required to be implemented. It should be noted that one step may require multiple genes such as for cofactor biosynthesis. In this regard, EG-utilizing microbes are useful as chassis for microbial cell factories from EG as the starting material since they do not require metabolic engineering for EG utilization.
Although EG catabolic pathways in diverse microbes have been characterized so far, their regulation seems to be less studied. For microbial conversion of EG to value-added chemicals, target compounds and/or metabolic intermediates might affect the regulation of EG catabolic enzymes unexpectedly as observed in the inhibition of EG utilization during β-KA production by P. putida (Werner et al. 2021). Thus, understanding the regulation of EG catabolic enzymes at both the transcriptional level and the post-translational level including allosteric regulation, substrate inhibition, product inhibition, and feedback inhibition would be required to improve the performance of microbial cell factories with EG and/or PET wastes as the starting materials.
In addition to natural EG-assimilating pathways, non-natural synthetic pathways for glycolaldehyde assimilation have been designed and validated for in vitro functionality (Yang et al. 2019; Mao et al. 2021; Scheffen et al. 2021). Notably, Wagner and co-workers demonstrated in vivo functionality of a synthetic pathway for the conversion of EG to acetyl-CoA in E. coli (Fig. 8) albeit at currently extremely small yields (Wagner et al. 2023a). Further design and improvement of non-natural pathways for EG utilization and a combination of those with natural pathways would be a future direction.
A non-natural pathway for carbon conserving conversion of ethylene glycol to acetyl-CoA. FucOI6L/L7V, I6L/L7V mutant of L-1,2-propandiol oxidoreductase from E. coli; FsaA, d-fructose 6-phosphate aldolase from E. coli; KdsD, d-arabinose 5-phosphate isomerase from E. coli; Rpe, d-ribulose 5-phosphate epimerase from E. coli; Pkt, phosphoketolase from Clostridium acetobutylicum; Pta, phosphate acetyltransferase from E. coli
Although microbial processes are expected to reduce energy costs and CO2 emission as compared to conventional chemical processes, most microbial processes have been designed to produce bio-chemicals or bio-fuels from edible biomass such as glucose, which would ultimately compete with food stocks (Dwi Prasetyo et al. 2020). In this regard, EG is a promising next-generation feedstock that can be derived from materials not competing food stocks, and EG preparation by means of sustainable process is rapidly developing. Thus, research on microbial conversion of EG to value-added chemicals will contribute to the development of a sustainable industry in parallel with research on microbial conversion of other potential next-generation feedstocks such as syngas (Kim et al. 2023), CO2 (Nisar et al. 2021), methanol (Zhang et al. 2019), and acetate (Kiefer et al. 2021).
References
Alruwaili A, Rashid GMM, Sodré V, Mason J, Rehman Z, Menakath AK, Cheung D, Brown SP, Bugg TDH (2023) Elucidation of microbial lignin degradation pathways using synthetic isotope-labelled lignin. RSC Chem Biol 4:47–55. https://doi.org/10.1039/D2CB00173J
Ayikpoe RS, Latham JA (2019) MftD catalyzes the formation of a biologically active redox center in the biosynthesis of the ribosomally synthesized and post-translationally modified redox cofactor mycofactocin. J Am Chem Soc 141:13582–13591. https://doi.org/10.1021/jacs.9b06102
Ayikpoe R, Govindarajan V, Latham JA (2019) Occurrence, function, and biosynthesis of mycofactocin. Appl Microbiol Biotechnol 103:2903–2912. https://doi.org/10.1007/s00253-019-09684-4
Baldomà L, Aguilar J (1988) Metabolism of L-fucose and L-rhamnose in Escherichia coli: aerobic-anaerobic regulation of L-lactaldehyde dissimilation. J Bacteriol 170:416–421. https://doi.org/10.1128/jb.170.1.416-421.1988
Bao T, Qian Y, Xin Y, Collins JJ, Lu T (2023) Engineering microbial division of labor for plastic upcycling. Nat Commun 14:5712. https://doi.org/10.1038/s41467-023-40777-x
Bartsch O, Hagemann M, Bauwe H (2008) Only plant-type (GLYK) glycerate kinases produce d-glycerate 3-phosphate. FEBS Lett 582:3025–3028. https://doi.org/10.1016/j.febslet.2008.07.038
Basen M, Müller V (2023) Editorial: Acetogens - from the origin of life to biotechnological applications. Front Microbiol 14. https://doi.org/10.3389/fmicb.2023.1186930
Bordel S, Martín-González D, Börner T, Muñoz R, Santos-Beneit F (2024) Genome-scale metabolic model of the versatile bacterium Paracoccus denitrificans Pd1222. mSystems 0:e01077-23. https://doi.org/10.1128/msystems.01077-23
Boronat A, Aguilar J (1979) Rhamnose-induced propanediol oxidoreductase in Escherichia coli: purification, properties, and comparison with the fucose-induced enzyme. J Bacteriol 140:320–326. https://doi.org/10.1128/jb.140.2.320-326.1979
Boronat A, Caballero E, Aguilar J (1983) Experimental evolution of a metabolic pathway for ethylene glycol utilization by Escherichia coli. J Bacteriol 153:134–139. https://doi.org/10.1128/jb.153.1.134-139.1983
Bourgade B, Humphreys CM, Millard J, Minton NP, Islam MA (2022) Design, analysis, and implementation of a novel biochemical pathway for ethylene glycol production in Clostridium Autoethanogenum. ACS Synth Biol 11:1790–1800. https://doi.org/10.1021/acssynbio.1c00624
Caballero E, Baldoma L, Ros J, Boronat A, Aguilar J (1983) Identification of lactaldehyde dehydrogenase and glycolaldehyde dehydrogenase as functions of the same protein in Escherichia coli. J Biol Chem 258:7788–7792. https://doi.org/10.1016/S0021-9258(18)32248-8
Chang Y, Wang A, Cronan J (1993) Molecular cloning, DNA sequencing, and biochemical analyses of Escherichia coli glyoxylate carboligase. An enzyme of the acetohydroxy acid synthase-pyruvate oxidase family. J Biol Chem 268:3911–3919. https://doi.org/10.1016/S0021-9258(18)53559-6
Chen YM, Tobin JF, Zhu Y, Schleif RF, Lin EC (1987) Cross-induction of the L-fucose system by L-rhamnose in Escherichia coli. J Bacteriol 169:3712–3719. https://doi.org/10.1128/jb.169.8.3712-3719.1987
Child J, Willetts A (1978) Microbial metabolism of aliphatic glycols bacterial metabolism of ethylene glycol. Biochim Biophys Acta 538:316–327. https://doi.org/10.1016/0304-4165(78)90359-8
Diao J, Hu Y, Tian Y, Carr R, Moon TS (2023) Upcycling of poly(ethylene terephthalate) to produce high-value bio-products. Cell Rep 42:111908. https://doi.org/10.1016/j.celrep.2022.111908
Diehl C, Gerlinger PD, Paczia N, Erb TJ (2023) Synthetic anaplerotic modules for the direct synthesis of complex molecules from CO2. Nat Chem Biol 19:168–175. https://doi.org/10.1038/s41589-022-01179-0
Donini E, Firrincieli A, Cappelletti M (2021) Systems biology and metabolic engineering of Rhodococcus for bioconversion and biosynthesis processes. Folia Microbiol 66:701–713. https://doi.org/10.1007/s12223-021-00892-y
Doughty C, Hayashi J, Guenther H (1966) Purification and properties of D-glycerate 3-kinase from Escherichia coli. J Biol Chem 241:568–572
Dwi Prasetyo W, Putra ZA, Bilad MR, Mahlia TMI, Wibisono Y, Nordin NAH, Wirzal MDH (2020) Insight into the sustainable integration of bio- and petroleum refineries for the production of fuels and chemicals. Polymers 12. https://doi.org/10.3390/polym12051091
Fan L, Zhao Y, Chen L, Chen J, Chen J, Yang H, Xiao Y, Zhang T, Chen J, Wang L (2023) Selective production of ethylene glycol at high rate via cascade catalysis. Nat Catal 6:585–595. https://doi.org/10.1038/s41929-023-00977-6
Franden MA, Jayakody LN, Li W-J, Wagner NJ, Cleveland NS, Michener WE, Hauer B, Blank LM, Wierckx N, Klebensberger J, Beckham GT (2018) Engineering Pseudomonas putida KT2440 for efficient ethylene glycol utilization. Metab Eng 48:197–207. https://doi.org/10.1016/j.ymben.2018.06.003
Frazão CJR, Wagner N, Rabe K, Walther T (2023) Construction of a synthetic metabolic pathway for biosynthesis of 2,4-dihydroxybutyric acid from ethylene glycol. Nat Commun 14:1931. https://doi.org/10.1038/s41467-023-37558-x
Fujiwara R, Sanuki R, Ajiro H, Fukui T, Yoshida S (2021) Direct fermentative conversion of poly(ethylene terephthalate) into poly(hydroxyalkanoate) by Ideonella sakaiensis. Sci Rep 11:19991. https://doi.org/10.1038/s41598-021-99528-x
Gaston LW, Stadtman ER (1963) Fermentation of ethylene glycol by Clostridium glycolicum, sp. n. J Bacteriol 85:356–362. https://doi.org/10.1128/jb.85.2.356-362.1963
Gonzalez CF, Taber WA, Zeitoun MA (1972) Biodegradation of ethylene glycol by a salt-requiring bacterium. Appl Microbiol 24:911–919. https://doi.org/10.1128/am.24.6.911-919.1972
Hachisuka S, Nishii T, Yoshida S (2021) Development of a targeted gene disruption system in the poly(ethylene terephthalate)-degrading bacterium Ideonella sakaiensis and its applications to PETase and MHETase genes. Appl Environ Microbiol 87:e00020–21. https://doi.org/10.1128/AEM.00020-21
Hachisuka S, Chong JF, Fujiwara T, Takayama A, Kawakami Y, Yoshida S (2022) Ethylene glycol metabolism in the poly(ethylene terephthalate)-degrading bacterium Ideonella sakaiensis. Appl Microbiol Biotechnol 106:7867–7878. https://doi.org/10.1007/s00253-022-12244-y
Hara H, Eltis LD, Davies JE, Mohn WW (2007) Transcriptomic analysis reveals a bifurcated terephthalate degradation pathway in Rhodococcus sp. strain RHA1. J Bacteriol 189:1641–1647. https://doi.org/10.1128/jb.01322-06
Hartmanis MGN, Stadtman TC (1986) Diol metabolism and diol dehydratase in Clostridium glycolicum. Arch Biochem Biophys 245:144–152. https://doi.org/10.1016/0003-9861(86)90198-0
Hua X, Cao R, Zhou X, Xu Y (2018) Integrated process for scalable bioproduction of glycolic acid from cell catalysis of ethylene glycol. Biores Technol 268:402–407. https://doi.org/10.1016/j.biortech.2018.08.021
Kataoka M, Sasaki M, Hidalgo A-RGD, Nakano M, Shimizu S (2001) Glycolic acid production using ethylene glycol-oxidizing microorganisms. Biosci Biotechnol Biochem 65:2265–2270. https://doi.org/10.1271/bbb.65.2265
Katsyv A, Müller V (2020) Overcoming energetic barriers in acetogenic C1 conversion. Front Bioeng Biotechnol 8. https://doi.org/10.3389/fbioe.2020.621166
Keltjens JT, Pol A, Reimann J, Op den Camp HJM (2014) PQQ-dependent methanol dehydrogenases: rare-earth elements make a difference. Appl Microbiol Biotechnol 98:6163–6183. https://doi.org/10.1007/s00253-014-5766-8
Kiefer D, Merkel M, Lilge L, Henkel M, Hausmann R (2021) From acetate to bio-based products: underexploited potential for industrial biotechnology. Trends Biotechnol 39:397–411. https://doi.org/10.1016/j.tibtech.2020.09.004
Kim J-Y, Lee M, Oh S, Kang B, Yasin M, Chang IS (2023) Acetogen and acetogenesis for biological syngas valorization. Biores Technol 384:129368. https://doi.org/10.1016/j.biortech.2023.129368
Kornberg H, Morris J (1965) The utilization of glycolate by Micrococcus denitrificans: the β-hydroxyaspartate pathway. Biochem J 95:577–586. https://doi.org/10.1042/bj0950577
Leow WR, Lum Y, Ozden A, Wang Y, Nam D-H, Chen B, Wicks J, Zhuang T-T, Li F, Sinton D, Sargent EH (2020) Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. Science 368:1228–1233. https://doi.org/10.1126/science.aaz8459
Li C, Zheng M, Wang A, Zhang T (2012) One-pot catalytic hydrocracking of raw woody biomass into chemicals over supported carbide catalysts: simultaneous conversion of cellulose, hemicellulose and lignin. Energy Environ Sci 5:6383–6390. https://doi.org/10.1039/C1EE02684D
Li W-J, Jayakody LN, Franden MA, Wehrmann M, Daun T, Hauer B, Blank LM, Beckham GT, Klebensberger J, Wierckx N (2019) Laboratory evolution reveals the metabolic and regulatory basis of ethylene glycol metabolism by Pseudomonas putida KT2440. Environ Microbiol 21:3669–3682. https://doi.org/10.1111/1462-2920.14703
Liang Y, Yu H (2021) Genetic toolkits for engineering Rhodococcus species with versatile applications. Biotechnol Adv 49:107748. https://doi.org/10.1016/j.biotechadv.2021.107748
Liew FE, Nogle R, Abdalla T, Rasor BJ, Canter C, Jensen RO, Wang L, Strutz J, Chirania P, De Tissera S, Mueller AP, Ruan Z, Gao A, Tran L, Engle NL, Bromley JC, Daniell J, Conrado R, Tschaplinski TJ, Giannone RJ, Hettich RL, Karim AS, Simpson SD, Brown SD, Leang C, Jewett MC, Köpke M (2022) Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale. Nat Biotechnol 40:335–344. https://doi.org/10.1038/s41587-021-01195-w
Liu F, Wang T, Yang W, Zhang Y, Gong Y, Fan X, Wang G, Lu Z, Wang J (2023) Current advances in the structural biology and molecular engineering of PETase. Front Bioeng Biotechnol 11. https://doi.org/10.3389/fbioe.2023.1263996
Lu Z, Cabiscol E, Obradors N, Tamarit J, Ros J, Aguilar J, Lin ECC (1998) Evolution of an Escherichia coli protein with increased resistance to oxidative stress*. J Biol Chem 273:8308–8316. https://doi.org/10.1074/jbc.273.14.8308
Lum Y, Huang JE, Wang Z, Luo M, Nam D-H, Leow WR, Chen B, Wicks J, Li YC, Wang Y, Dinh C-T, Li J, Zhuang T-T, Li F, Sham T-K, Sinton D, Sargent EH (2020) Tuning OH binding energy enables selective electrochemical oxidation of ethylene to ethylene glycol. Nat Catal 3:14–22. https://doi.org/10.1038/s41929-019-0386-4
Mao Y, Yuan Q, Yang X, Liu P, Cheng Y, Luo J, Liu H, Yao Y, Sun H, Cai T, Ma H (2021) Non-natural aldol reactions enable the design and construction of novel one-carbon assimilation pathways in vitro. Front Microbiol 12. https://doi.org/10.3389/fmicb.2021.677596
Montella C, Bellsolell L, Pérez-Luque R, Badía J, Baldoma L, Coll M, Aguilar J (2005) Crystal structure of an iron-dependent group III dehydrogenase that interconverts L-lactaldehyde and L-1,2-propanediol in Escherichia coli. J Bacteriol 187:4957–4966. https://doi.org/10.1128/jb.187.14.4957-4966.2005
Mückschel B, Simon O, Klebensberger J, Graf N, Rosche B, Altenbuchner J, Pfannstiel J, Huber A, Hauer B (2012) Ethylene glycol metabolism by Pseudomonas putida. Appl Environ Microbiol 78:8531–8539. https://doi.org/10.1128/AEM.02062-12
Narancic T, Salvador M, Hughes GM, Beagan N, Abdulmutalib U, Kenny ST, Wu H, Saccomanno M, Um J, O’Connor KE, Jiménez JI (2021) Genome analysis of the metabolically versatile Pseudomonas umsongensis GO16: the genetic basis for PET monomer upcycling into polyhydroxyalkanoates. Microb Biotechnol 14:2463–2480. https://doi.org/10.1111/1751-7915.13712
Nisar A, Khan S, Hameed M, Nisar A, Ahmad H, Mehmood SA (2021) Bio-conversion of CO2 into biofuels and other value-added chemicals via metabolic engineering. Microbiol Res 251:126813. https://doi.org/10.1016/j.micres.2021.126813
Njau RK, Herndon CA, Hawes JW (2000) Novel β-hydroxyacid dehydrogenases in Escherichia coli and Haemophilus influenzae*. J Biol Chem 275:38780–38786. https://doi.org/10.1074/jbc.M007432200
Palm GJ, Reisky L, Böttcher D, Müller H, Michels EAP, Walczak MC, Berndt L, Weiss MS, Bornscheuer UT, Weber G (2019) Structure of the plastic-degrading Ideonella sakaiensis MHETase bound to a substrate. Nat Commun 10:1717. https://doi.org/10.1038/s41467-019-09326-3
Panda S, Zhou JFJ, Feigis M, Harrison E, Ma X, Fung Kin Yuen V, Mahadevan R, Zhou K (2023) Engineering Escherichia coli to produce aromatic chemicals from ethylene glycol. Metab Eng 79:38–48. https://doi.org/10.1016/j.ymben.2023.06.012
Pandit AV, Harrison E, Mahadevan R (2021) Engineering Escherichia coli for the utilization of ethylene glycol. Microb Cell Fact 20:22. https://doi.org/10.1186/s12934-021-01509-2
Pang J, Zheng M, Wang A, Zhang T (2011) Catalytic hydrogenation of corn stalk to ethylene glycol and 1,2-propylene glycol. Ind Eng Chem Res 50:6601–6608. https://doi.org/10.1021/ie102505y
Parales RE, Harwood CS (1992) Characterization of the genes encoding beta-ketoadipate: succinyl-coenzyme A transferase in Pseudomonas putida. J Bacteriol 174:4657–4666. https://doi.org/10.1128/jb.174.14.4657-4666.1992
Parales RE, Harwood CS (1993) Regulation of the pcaIJ genes for aromatic acid degradation in Pseudomonas putida. J Bacteriol 175:5829–5838. https://doi.org/10.1128/jb.175.18.5829-5838.1993
Pellicer MT, Badía J, Aguilar J, Baldomà L (1996) glc locus of Escherichia coli: characterization of genes encoding the subunits of glycolate oxidase and the glc regulator protein. J Bacteriol 178:2051–2059. https://doi.org/10.1128/jb.178.7.2051-2059.1996
Puehringer S, Metlitzky M, Schwarzenbacher R (2008) The pyrroloquinoline quinone biosynthesis pathway revisited: a structural approach. BMC Biochem 9:8. https://doi.org/10.1186/1471-2091-9-8
Rodionova IA, Hosseinnia A, Kim S, Goodacre N, Zhang L, Zhang Z, Palsson B, Uetz P, Babu M, Saier MH (2023) E. Coli allantoinase is activated by the downstream metabolic enzyme, glycerate kinase, and stabilizes the putative allantoin transporter by direct binding. Sci Rep 13:7345. https://doi.org/10.1038/s41598-023-31812-4
Round JW, Robeck LD, Eltis LD (2021) An integrative toolbox for synthetic biology in Rhodococcus. ACS Synth Biol 10:2383–2395. https://doi.org/10.1021/acssynbio.1c00292
Salusjärvi L, Havukainen S, Koivistoinen O, Toivari M (2019) Biotechnological production of glycolic acid and ethylene glycol: current state and perspectives. Appl Microbiol Biotechnol 103:2525–2535. https://doi.org/10.1007/s00253-019-09640-2
Sasoh M, Masai E, Ishibashi S, Hara H, Kamimura N, Miyauchi K, Fukuda M (2006) Characterization of the terephthalate degradation genes of Comamonas sp. strain E6. Appl Environ Microbiol 72:1825–1832. https://doi.org/10.1128/AEM.72.3.1825-1832.2006
Satapathy A, Gadge ST, Bhanage BM (2018) Synthesis of ethylene glycol from syngas via oxidative double carbonylation of ethanol to diethyl oxalate and its subsequent hydrogenation. ACS Omega 3:11097–11103. https://doi.org/10.1021/acsomega.8b01307
Scheffen M, Marchal DG, Beneyton T, Schuller SK, Klose M, Diehl C, Lehmann J, Pfister P, Carrillo M, He H, Aslan S, Cortina NS, Claus P, Bollschweiler D, Baret J-C, Schuller JM, Zarzycki J, Bar-Even A, Erb TJ (2021) A new-to-nature carboxylation module to improve natural and synthetic CO2 fixation. Nat Catal 4:105–115. https://doi.org/10.1038/s41929-020-00557-y
Schuchmann K, Schmidt S, Lopez AM, Kaberline C, Kuhns M, Lorenzen W, Bode HB, Joos F, Müller V (2015) Nonacetogenic growth of the acetogen Acetobacterium woodii on 1,2-propanediol. J Bacteriol 197:382–391. https://doi.org/10.1128/jb.02383-14
Shimizu T, Suzuki K, Inui M (2024) A mycofactocin-associated dehydrogenase is essential for ethylene glycol metabolism by Rhodococcus jostii RHA1. Appl Microbiol Biotechnol 108:58. https://doi.org/10.1007/s00253-023-12966-7
Sun Y-L, Chai G-L (2022) Direct synthesis of ethylene glycol from syngas. ChemistrySelect 7:e202103642. https://doi.org/10.1002/slct.202103642
Tamura J, Ono A, Sugano Y, Huang C, Nishizawa H, Mikoshiba S (2015) Electrochemical reduction of CO2 to ethylene glycol on imidazolium ion-terminated self-assembly monolayer-modified au electrodes in an aqueous solution. Phys Chem Chem Phys 17:26072–26078. https://doi.org/10.1039/C5CP03028E
Tan HT, Chek MF, Neoh SZ, Ang SL, Yoshida S, Hakoshima T, Sudesh K (2022) Characterization of the polyhydroxyalkanoate (PHA) synthase from Ideonella sakaiensis, a bacterium that is capable of degrading and assimilating poly(ethylene terephthalate). Polym Deg Stab 206:110160. https://doi.org/10.1016/j.polymdegradstab.2022.110160
te Molder TDJ, Kersten SRA, Lange JP, Ruiz MP (2021) Ethylene glycol from lignocellulosic biomass: impact of lignin on catalytic hydrogenolysis. Ind Eng Chem Res 60:7043–7049. https://doi.org/10.1021/acs.iecr.1c01063
Tiso T, Narancic T, Wei R, Pollet E, Beagan N, Schröder K, Honak A, Jiang M, Kenny ST, Wierckx N, Perrin R, Avérous L, Zimmermann W, O’Connor K, Blank LM (2021) Towards bio-upcycling of polyethylene terephthalate. Metab Eng 66:167–178. https://doi.org/10.1016/j.ymben.2021.03.011
Trifunović D, Schuchmann K, Müller V (2016) Ethylene glycol metabolism in the acetogen Acetobacterium woodii. J Bacteriol 198:1058–1065. https://doi.org/10.1128/jb.00942-15
von Borzyskowski LS, Bernhardsgrütter I, Erb TJ (2020) Biochemical unity revisited: microbial central carbon metabolism holds new discoveries, multi-tasking pathways, and redundancies with a reason. Biol Chem 401:1429–1441. https://doi.org/10.1515/hsz-2020-0214
von Borzyskowski LS, Schulz-Mirbach H, Castellanos MT, Severi F, Gómez-Coronado PA, Paczia N, Glatter T, Bar-Even A, Lindner SN, Erb TJ (2023) Implementation of the β-hydroxyaspartate cycle increases growth performance of Pseudomonas putida on the PET monomer ethylene glycol. Metab Eng 76:97–109. https://doi.org/10.1016/j.ymben.2023.01.011
Schada von Borzyskowski L, Severi F, Krüger K, Hermann L, Gilardet A, Sippel F, Pommerenke B, Claus P, Cortina NS, Glatter T, Zauner S, Zarzycki J, Fuchs BM, Bremer E, Maier UG, Amann RI, Erb TJ (2019) Marine Proteobacteria metabolize glycolate via the β-hydroxyaspartate cycle. Nature 575:500–504. https://doi.org/10.1038/s41586-019-1748-4
Wagner N, Bade F, Straube E, Rabe K, Frazão CJR, Walther T (2023a) In vivo implementation of a synthetic metabolic pathway for the carbon-conserving conversion of glycolaldehyde to acetyl-CoA. Front Bioeng Biotechnol 11. https://doi.org/10.3389/fbioe.2023.1125544
Wagner N, Wen L, Frazão CJR, Walther T (2023b) Next-generation feedstocks methanol and ethylene glycol and their potential in industrial biotechnology. Biotechnol Adv 69:108276. https://doi.org/10.1016/j.biotechadv.2023.108276
Wei G, Yang X, Gan T, Zhou W, Lin J, Wei D (2009) High cell density fermentation of Gluconobacter oxydans DSM 2003 for glycolic acid production. J Ind Microbiol Biotechnol 36:1029–1034. https://doi.org/10.1007/s10295-009-0584-1
Werner AZ, Clare R, Mand TD, Pardo I, Ramirez KJ, Haugen SJ, Bratti F, Dexter GN, Elmore JR, Huenemann JD, Peabody GL, Johnson CW, Rorrer NA, Salvachúa D, Guss AM, Beckham GT (2021) Tandem chemical deconstruction and biological upcycling of poly(ethylene terephthalate) to β-ketoadipic acid by Pseudomonas putida KT2440. Metab Eng 67:250–261. https://doi.org/10.1016/j.ymben.2021.07.005
Yang X, Yuan Q, Luo H, Li F, Mao Y, Zhao X, Du J, Li P, Ju X, Zheng Y, Chen Y, Liu Y, Jiang H, Yao Y, Ma H, Ma Y (2019) Systematic design and in vitro validation of novel one-carbon assimilation pathways. Metab Eng 56:142–153. https://doi.org/10.1016/j.ymben.2019.09.001
Yoshida S, Hiraga K, Takehana T, Taniguchi I, Yamaji H, Maeda Y, Toyohara K, Miyamoto K, Kimura Y, Oda K (2016) A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 351:1196–1199. https://doi.org/10.1126/science.aad6359
Zavarise A, Sridhar S, Kiema T-R, Wierenga RK, Widersten M (2023) Structures of lactaldehyde reductase, FucO, link enzyme activity to hydrogen bond networks and conformational dynamics. FEBS J 290:465–481. https://doi.org/10.1111/febs.16603
Zelcbuch L, Razo-Mejia M, Herz E, Yahav S, Antonovsky N, Kroytoro H, Milo R, Bar-Even A (2015) An in vivo metabolic approach for deciphering the product specificity of glycerate kinase proves that both E. Coli’s glycerate kinases generate 2-phosphoglycerate. PLoS ONE 10:1–7. https://doi.org/10.1371/journal.pone.0122957
Zhang X, Zhang B, Lin J, Wei D (2015) Oxidation of ethylene glycol to glycolaldehyde using a highly selective alcohol dehydrogenase from Gluconobacter oxydans. J Mol Catal B Enzym 112:69–75. https://doi.org/10.1016/j.molcatb.2014.12.006
Zhang M, Yuan X, Zhang C, Zhu L, Mo X, Chen W, Yang S (2019) Bioconversion of methanol into value-added chemicals in native and synthetic methylotrophs. Curr Issues Mol Biol 33:225–236. https://doi.org/10.21775/cimb.033.225
Funding
This work was supported by NEDO Moonshot R&D.
Author information
Authors and Affiliations
Contributions
Conceptualization: MI, TS; literature search: TS; writing: TS, MI; writing review and editing: MI; project administration: MI. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or vertebrates performed by any of the authors..
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shimizu, T., Inui, M. Novel aspects of ethylene glycol catabolism. Appl Microbiol Biotechnol 108, 369 (2024). https://doi.org/10.1007/s00253-024-13179-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13179-2