Abstract
Saccharomyces cerevisiae is a widely used strain for ethanol fermentation; meanwhile, efficient utilization of glucose could effectively promote ethanol production. The PFK1 gene is a key gene for intracellular glucose metabolism in S. cerevisiae. Our previous work suggested that although deletion of the PFK1 gene could confer higher oxidative tolerance to S. cerevisiae cells, the PFK1Δ strain was prone to contamination by other microorganisms. High interspecies microbial competition ability is vital for the growth and survival of microorganisms in co-cultures. The result of our previous studies hinted us a reasonable logic that the EMP (i.e., the Embden-Meyerhof-Parnas pathway, the glycolytic pathway) key gene PFK1 could be involved in regulating interspecies competitiveness of S. cerevisiae through the regulation of glucose utilization and ethanol production efficiency. The results suggest that under 2% and 5% glucose, the PFK1Δ strain showed slower growth than the S288c wild-type and TDH1Δ strains in the lag and exponential growth stages, but realized higher growth in the stationary stage. However, relative high supplement of glucose (10%) eliminated this phenomenon, suggesting the importance of glucose in the regulation of PFK1 in yeast cell growth. Furthermore, during the lag growth phase, the PFK1Δ strain displayed a decelerated glucose consumption rate (P < 0.05). The expression levels of the HXT2, HXT5, and HXT6 genes decreased by approximately 0.5-fold (P < 0.05) and the expression level of the ZWF1 exhibited a onefold increase in the PFK1Δ strain compared to that in the S. cerevisiae S288c wild-type strain (P < 0.05).These findings suggested that the PFK1 inhibited the uptake and utilization of intracellular glucose by yeast cells, resulting in a higher amount of residual glucose in the medium for the PFK1Δ strain to utilize for growth during the reverse overshoot stage in the stationary phase. The results presented here also indicated the potential of ethanol as a defensive weapon against S. cerevisiae. The lower ethanol yield in the early stage of the PFK1Δ strain (P < 0.001) and the decreased expression levels of the PDC5 and PDC6 (P < 0.05), which led to slower growth, resulted in the strain being less competitive than the wild-type strain when co-cultured with Escherichia coli. The lower interspecies competitiveness of the PFK1Δ strain further promoted the growth of co-cultured E. coli, which in turn activated the ethanol production efficiency of the PFK1Δ strain to antagonize it from E. coli at the stationary stage. The results presented clarified the regulation of the PFK1 gene on the growth and interspecies microbial competition behavior of S. cerevisiae and would help us to understand the microbial interactions between S. cerevisiae and other microorganisms.
Key points
• PFK1Δ strain could realize reverse growth overshoot at the stationary stage
• PFK1 deletion decreased ethanol yield and interspecific competitiveness
• Proportion of E. coli in co-culture affected ethanol yield capacity of yeast cells
Similar content being viewed by others
Introduction
Saccharomyces cerevisiae is a commonly used species for industrial fermentation (Madhavan et al. 2021), such as the production of beverages, bioethanol, and other chemicals (Lacerda et al. 2020). It is often used in co-fermentation with other microorganisms to promote fermentation efficiency. For example, to add a unique flavor, fermentation by S. cerevisiae is usually co-cultured with other microorganisms (Yılmaz and Gökmen 2021; Soden et al. 2008), such as non-Saccharomyces yeast and lactic acid bacteria (Sieuwerts et al. 2018; Jin et al. 2021); meanwhile, and to improve the production efficiency, Acremonium cellulolyticus C-1 was co-cultured with S. cerevisiae for realizing the simultaneous saccharification and fermentation of ethanol by a one-pot method without any pretreatment or addition of cellulase (Park et al. 2012).
In mixed fermentation, a microsystem is formed between different microorganisms (Pandhal and Noirel 2014). There are many complex interactions between microorganisms in microsystems like these, including competition, mutualism, amensalism, and predation (Zengler and Zaramela 2018). For example, in the sesame wine fermentation process, although there is a competitive relationship among the five dominant microorganisms (i.e., S. cerevisiae, Bacillus licheniformis, Pichia membranaefaciens, Bacillus amyloliquefaciens, and Issatchenkia orientalis), their cooperation also facilitates the production of most flavor compounds (Wu et al. 2014). Similarly, our previous research also indicated that co-cultivated Lactobacillus plantarum not only inhibits the growth of S. cerevisiae (Dong et al. 2015), but also enhances the oxidative and ethanol tolerance of yeast cells (He et al. 2021; Kang et al. 2021).
Microbial metabolic activity may modulate the cellular behavior such as tolerance (Kotte et al. 2010; Heinemann and Sauer 2010). Meanwhile, co-cultured L. plantarum associated with regulation of carbohydrate metabolism (Dong et al. 2015) and higher oxidative and ethanol tolerance of S. cerevisiae also hinted at a possible correlation between central carbon metabolism and tolerance in yeast cells. To test this hypothesis, five different mutant strains with deletion of the EMP (i.e., the Embden-Meyerhof-Parnas pathway, the glycolytic pathway) key genes HXK1, ENO1, TDH1, PFK1, and FBP1 were constructed from S. cerevisiae S288c strain using the CRISPR-Cas9 strategy, and the results suggested that the PFK1Δ strain always showed slower growth in the early growth stage but reversed overshoot in the later stage in comparison to the wild strain and the other four mutant strains (Kang 2022). Interestingly, under the same culture conditions, the PFK1Δ strain was more susceptible to invasion by other microorganisms (i.e., Escherichia coli and Acinetobacter nosocomialis) compared to that of the wild-type strain and the other four mutant strains. These results preliminarily suggest the PFK1 gene regulates on the interspecies competition behavior of S. cerevisiae.
Microorganisms have evolved various weapons, both defensive and offensive, in their evolutionary process to enhance their survival capabilities and maximize their own benefits (Granato et al. 2019). For instance, bacteria in the soil may compete for scarce resources by either manufacturing toxins or enhancing their motility (Hibbing et al. 2010; Ghoul and Mitri 2016). Streptomycin produced by the filamentous bacterium Streptomyces griseus can be used as a defensive and offensive weapon to kill some susceptible species to prevent their invasion, thereby improving the competitiveness of S. griseus (Westhoff et al. 2020). Similar to the “The Red Queen hypothesis” (Strotz et al. 2018), interspecific relationships can also promote the evolution of species under certain environmental conditions. In a similar vein, S. cerevisiae employs strategies to enhance its competitiveness, either proactively or passively, while it coexists with other microorganisms. When the benefits of such strategies are greater than the input, it will continue to evolve more favorable strains in this direction. Meanwhile, ethanol has been effectively used in disinfection and sterilization in many fields (Mathew and Goyal 2023). Ethanol, as a metabolite of S. cerevisiae, exerted inhibitory effects on microorganism growth at high concentrations. Elevated ethanol concentrations could disrupt cell membrane integrity and functionality, thereby interfering with intracellular metabolism and compromising cell physiological activities (Patra et al. 2006). It is worth noting that different microorganisms may possess varying degrees of ethanol tolerance (Ingram 1990), which could influence the competitive dynamics between S. cerevisiae and other microorganisms. In nature, S. cerevisiae can tolerate higher ethanol concentrations than other microorganisms (Pina et al. 2004; Arroyo-López et al. 2010) to enable its survival and proliferation even in environments with relatively high ethanol concentrations, suggesting that ethanol might serve as a defense weapon for yeast to improve its viability and antagonize other microorganisms to maximize its benefits during co-existence. As a result, it can be logically inferred that a greater ethanol concentration will lead to improved competitiveness and survivability of S. cerevisiae when coexisting with other microorganisms, whereas a lower ethanol concentration will be less favorable for its survival. Based on our previous findings, we can propose a plausible hypothesis that the PFK1 gene plays a crucial role in regulating the ethanol production ability of S. cerevisiae by controlling its glucose uptake and utilization, which ultimately affects the interspecies competitiveness of S. cerevisiae.
Here, the growth under different concentrations of glucose supplements, glucose metabolism, and ethanol production of three strains (i.e., S288c wild-type, PFK1Δ, and TDH1Δ strains) were measured, and the expression of related metabolic genes between the S288c wild-type and PFK1Δ strains was compared. Moreover, the E. coli co-culture-associated biomass change and the expression of genes involved in ethanol synthesis of the S288c wild-type and PFK1Δ strain were also determined. The results presented here will help us understand the regulation of the PFK1 gene on the growth and interspecies microbial competition behavior of S. cerevisiae.
Materials and methods
Strains, media, and cultural conditions
S. cerevisiae S288c strain (ATCC 204508) was purchased from the Institute of Microbiology of the Chinese Academy of Sciences (IMCAS). The PFK1Δ and TDH1Δ strains were constructions from the S288c strain using the CRISPR-Cas9 strategy. S. cerevisiae S288c wild-type, PFK1Δ, and TDH1Δ strains were cultured in 250-ml conical flasks containing 100-ml YPD broth (2% glucose, 2% peptone, and 1% yeast extract) at 30 °C and shaken at 150 rpm. E. coli BL21 (DE3) was also purchased from Institute of Microbiology of the Chinese Academy of Sciences (IMCAS), and cultured in 250-ml conical flasks containing 100 ml of LB broth (1% peptone, 0.5% yeast extract, 1% sodium chloride) at 37 °C and shaken at 180 rpm.
Determination of growth differences of three yeast strains supplied with various concentrations of glucose
The S288c, PFK1Δ, and TDH1Δ strains were inoculated into 100-ml YPD medium with an inoculation cell density of 4 × 105 cfu/ml (i.e., colony forming units per milliliter), and the OD600 value was measured by using an UV spectrophotometer after sampling every 2 h to determine the growth curve. The concentration of glucose in the medium was increased to 5% and 10%, respectively, to observe the impact of different carbon sources on microbial metabolism and growth. Samples were taken every 4 h to measure the OD600 value. The growth rate was calculated using the following formula:
X2 and X1 refer to the OD600 values of cell cultures at culture time (t) t1 and t2, respectively.
Determination of the contents of extracellular and intracellular glucose
The contents of extracellular and intracellular glucose in yeast during cultivation were determined by HPLC (high-performance liquid chromatography; Shimadzu, i-Series, Kyoto, Japan) analysis. The chromatographic column was Bio-Rad HPX-87H (9 μm, 300 × 7.8 mm, CA, USA), the mobile phase was 7 mmol/l H2SO4, the column temperature was 55 °C, and the flow rate was 0.6 ml/min. To determine the extracellular glucose content, the initial inoculation densities of the three strains were all 4 × 105 cfu/ml, and 1.5 ml of cultured suspension was collected from 12 h, 14 h, 16 h, and 20 h of cultivation. After centrifugation, the supernatant was added into a liquid injection vial through a 0.22-μm filter for HPLC analysis. To measure the intracellular glucose levels, a 50-ml culture sample was collected from the yeast cells after 20 h of cultivation. The supernatant was then removed by centrifugation, and the cells were washed 3 times with 1 × PBS before being resuspended in 25 ml of deionized water. Repeated freeze–thaw cycles and ultrasonic crushing (ultrasonic power 400 W, radiation time 11 s, total time 14.5 min) were performed to break the yeast cell wall. After centrifugation, the supernatant was filtered through a 0.22-μm filter for HPLC analysis.
Moreover, the extracellular ethanol contents at the 12, 14, 16, and 20 h of cultivation were also determined by HPLC analysis.
Determination of expressions of genes involved in the hexose transporter family, pentose phosphate pathway, and ethanol synthesis
Key genes involved in the hexose transporter family, pentose phosphate pathway, and ethanol synthesis were chosen to determine the PFK1 deletion-associated expression level changes. Yeast cells were collected after 10 and 20 h of cultivation. Primers used in this work were designed using Primer Premier 5 (PREMIER Biosoft International, San Francisco, CA, USA) and are listed in Table 1. RNA extraction, reverse transcription, and qRT-PCR analysis were performed following the procedure reported in our previous work (Dong et al. 2015).
Evaluation of the potential ability of ethanol to antagonize E. coli
To test whether ethanol could be used as a defensive weapon for yeast cells, physically quarantined S. cerevisiae secreted ethanol content in the presence or absence of E. coli was determined (Supplemental Fig. S1A). A physical quarantine system was constructed using three minimum inhibitory concentration (MIC) bottles that were separated from each other with a 0.45-μm filter membrane. In the physical quarantine system, microorganisms cultured in different bottles cannot touch each other, while the secreted molecules (i.e., ethanol) can freely permeate the filter membrane and enter the other bottles. S. cerevisiae (4 × 105 cfu/ml) was inoculated into the middle bottle, and E. coli (1 × 108 cfu/ml) was inoculated into the left bottle, while the right side bottle without any inoculation was considered as the blank control. The ethanol content in each bottle was determined through HPLC analysis after 10 or 20 h of cultivation. Meanwhile, to further eliminate the effects of S. cerevisiae cell wall and other substances on E. coli, the yeast cell wall was degraded and the supernatant was replaced. Approximately 5 × 107 cells from S. cerevisiae suspension cultured in the exponential phase were centrifuged and treated with yeast wall-breaking enzyme solution (Solarbio, Beijing, China) at 30 °C for 2 h. Protoplasts were inoculated into an LB medium containing E. coli and cultured for 1 h. The number of E. coli was calculated using an LB agar medium containing 10 mg/l natamycin (Liu et al. 2017). Normal S. cerevisiae cells were used as the control. In the other group, E. coli culture was suspended and centrifuged to separate the precipitate. This precipitate was then added to the supernatant of S. cerevisiae and the YPD liquid medium containing a certain concentration of ethanol separately (i.e., ethanol produced by S. cerevisiae cultured for 10 h). E. coli treated with YPD was used as a control. The growth of E. coli was compared to the LB agar medium counting method after culturing at 37 °C for 1 h.
Finally, according to the ethanol concentration produced by S. cerevisiae at 10, 14, 16, and 20 h, different concentrations of ethanol solution were prepared and added to E. coli cultured to the exponential phase for 1 h. The agar plate method was used to count the diluted bacterial suspension, and the effects of varying ethanol concentrations on the growth of E. coli were compared.
Determination of the growth of S. cerevisiae and E. coli in the co-culture
To evaluate the susceptibility of the PFK1Δ strain to other microorganisms (i.e., E. coli in this study), one type of S. cerevisiae strain (i.e., S288c wild-type, PFK1Δ type, or TDH1Δ type) was co-inoculated with E. coli into the YPD medium, and the growth of yeast and E. coli were, respectively, determined every 4 h. To simulate the co-culture system and ensure that the growth was easy to determine, the inoculation cell densities of S. cerevisiae and E. coli were 4 × 105 cfu/ml and 1 × 106 cfu/ml, respectively. The number of S. cerevisiae cells was determined by microscopy, and the biomass of E. coli was measured by counting the number of cfu on LB agar medium containing 10 mg/l natamycin after 24 h of culture (Liu et al. 2017).
Moreover, to evaluate the E. coli co-culture associated with ethanol production difference between the two yeast strains (i.e., S288c wild-type and PFK1Δ type), key genes involved in ethanol synthesis were chosen to determine their expression level changes. To ensure the accuracy of the expression level analysis of yeast genes, we removed E. coli cells from the co-culture system using the G5 (2–4-μm pore diameter) sand core funnel, following our previous method (Dong et al. 2015). Following the collection of yeast cells from the funnel, RNA was obtained for reverse transcription, and gene expression was evaluated through quantitative reverse transcription polymerase chain reaction (qRT-PCR).
Statistical analysis
An independent-sample T test was performed on specific metabolites and genes using SPSS software (version 20.0; IBM Corp., Armonk, NY USA), to assess the statistical significance of the metabolic changes, and the error bars correspond to standard error. All experiments were run in triplicate to quintuplicate. Differences showing P-values less than 0.05 were considered statistically.
Results
Growth differences among S. cerevisiae S288c wild-type, PFK1Δ, and TDH1Δ strains
The PFK1Δ strain showed slower growth than the S288c wild-type and the TDH1Δ strain in the lag and exponential growth stages but showed higher growth in the stationary stage at of 2% glucose (Fig. 1 A and B). The growth of the TDH1Δ mutant strain was not noticeably different from that of the wild-type strain (Fig. 1A). The three strains showed similar growth patterns when the glucose concentration in the medium was increased to 5%, as it was under 2% glucose concentration (Fig. 1C). When the glucose concentration in the medium was further increased to 10%, although lower growth biomass of the PFK1Δ strain in the lag and exponential stages still existed, its higher growth biomass in the stationary stage was not detected (Fig. 1E). Consequently, varying concentrations of extracellular and intracellular glucose can influence the proliferation of the three microbial strains. The supply of carbon sources in the later stages of growth is essential to realize growth reversal.
Growth curve of the Saccharomyces cerevisiae S288c wild-type, PFK1Δ, and TDH1Δ strains under different sugar concentrations. A The growth curve of the three strains was determined every 2 h under a 2% glucose concentration in the culture medium, intending to identify the range of inflection points. B The growth rate of the three strains under a 2% glucose concentration. C Under the condition of 5% glucose concentration in the culture medium, to determine whether there was still an anti-overshoot phenomenon, the growth curves of three strains were obtained by measuring the OD600 value every 4 h. D The growth rate of the three strains under a 5% glucose concentration. E Growth curves of three strains under the condition of medium containing 10% glucose concentration. OD600 values were measured every 4 h. F The growth rate of the three strains under a 10% glucose concentration
Differences in intracellular and extracellular glucose contents, and ethanol yield among S. cerevisiae S288c wild-type, PFK1Δ, and TDH1Δ strains
Two time points were chosen — one before and one after a critical point — in order to monitor glucose utilization and ethanol production, and to validate the impact of varying glucose levels on cell growth. In the exponential phase (i.e., 10 and 14 h of cultivation), the extracellular glucose level of PFK1Δ (7.33 mg/ml) strain was higher than that of the S288c (2.60 mg/ml) and TDH1Δ (3.08 mg/ml) strains (P < 0.05) (Fig. 2 A and B). In the early stationary phase (i.e., 16 h of cultivation), only the PFK1Δ strain retained extracellular glucose with a concentration of 0.9 mg/ml (Fig. 2C). The yield of ethanol produced by the PFK1Δ strain was lower than that produced by S288c and TDH1Δ strains (P < 0.05) (Fig. 2 A and B). However, the ethanol fermentation concentration of the three strains could reach 10 mg/ml at 20 h (Fig. 2D). Meanwhile, the glucose content of the PFK1Δ strain (1.3 mg/g) was higher than that of the S288c (0.36 mg/g) and TDH1Δ strains (0.49 mg/g) after 20 h of cultivation (P < 0.05) (Fig. 2E).
Comparison of sugar consumption and ethanol yield of three strains at different time points. The concentration of extracellular glucose and ethanol of each of the three strains was measured at 10 h of incubation (A), at 14 h of incubation (B), at 16 h of incubation (C), and at 20 h of incubation (D). E Intracellular glucose content of three strains after the cell wall broken at 20 h. *P < 0.05 compared to the S288c wild-type strain. **P < 0.01 compared to the S288c wild-type strain. ***P < 0.001 compared to the wild-type S288c strain
Effect of PFK1 gene deletion on hexose transporter family, pentose phosphate pathway, and ethanol synthesis gene expression in S. cerevisiae
HXT2, HXT5, and HXT6/7, which are the key genes involved in glucose transport in S. cerevisiae, suggest low, intermediate, and high affinity for glucose, respectively (Buziol et al. 2008; Ye et al. 2001). After 10 h of cultivation, the PFK1Δ strain exhibited a 0.5-fold decrease in the expression levels of HXT2, HXT5, and HXT6 compared to the S. cerevisiae S288c wild-type strain (P < 0.05) (Fig. 3A). However, the expression of ZWF1, the key gene in the pentose phosphate pathway (HMP) (DangThu et al. 2020), increased by 0.8-fold in PFK1Δ strain after 10 h of cultivation (P < 0.05) (Fig. 3B). Although the expression of HXT2 and HXT5 did not change or even decreased at 20 h of cultivation, the expression levels of HXT6 and HXT7, which have a high affinity for glucose, exhibited 0.6-fold and onefold increases, respectively. (P < 0.05) (Fig. 3C). At this time, the expression level of ZWF1 in PFK1Δ strain still exhibited a onefold increase (P < 0.05) (Fig. 3D).
Effect of PFK1 gene deletion on hexose transporter family, pentose phosphate pathway, and ethanol synthesis gene expression in S. cerevisiae. Comparison of expression levels of key genes HXT2, HXT5, HXT6, and HXT7 controlling glucose transport in the S288c wild-type strain and the PFK1Δ strain at 10 h (A) and 20 h (C). Comparison of expression levels of ZWF1, a key gene controlling HMP pathway, in S288c wild-type and PFK1Δ strains at 10 h (B) and 20 h (D). Comparison of expression levels of key genes ADH1, PDC1, PDC5, and PDC6 controlling ethanol synthesis in the S288c wild-type and the PFK1Δ strains at 8 h (E) and 16 h (F). *P < 0.05 compared to the S288c wild-type strain. **P < 0.01 compared to the S288c wild-type strain
Pyruvate decarboxylase ( encoded by PDC) and alcohol dehydrogenase (from gene ADH) were the key enzymes involved in ethanol synthesis (Strommer and Garabagi 2008). However, ADH is more critical than PDC for ethanol biosynthesis (Ke et al. 2015). Here, the PFK1 gene deletion was associated with lower expressions of PDC5 and PDC6 (P < 0.05) (Fig. 3E) in the early stage of growth. During the stationary growth stage, although the expression of the PDC5 gene was upregulated in PFK1Δ strain (P < 0.05) (Fig. 3F), the down-regulation of the ADH1 gene (P < 0.001) limited the ethanol production efficiency of PFK1Δ strain (Fig. 3F).
Antagonistic effect of ethanol on E. coli
After co-cultivation with intact yeast cells and S. cerevisiae protoplasts, the E. coli biomass of both groups was about 1.7 × 109 cfu/ml, which was significantly lower than that of 2.23 × 109 cfu/ml of the control group (P < 0.01) (Supplemental Fig. S1B). Through the physical isolation of MIC bottles, the ethanol concentration of the experimental group inoculated with E. coli was 0.292 mg/ml at 10 h, which was significantly higher than that of the uninoculated blank group (0. 04 mg/ml) (P < 0.001) (Supplemental Fig. S2). Simultaneously, the concentration of ethanol in separately cultured E. coli (0.133 mg/ml) was determined to eliminate self-interference. After using the supernatant of cultured S. cerevisiae and the YPD liquid medium containing 2.23 mg/ml ethanol (i.e., the ethanol concentration in the supernatant of S. cerevisiae cultured for 10 h) to replace the supernatant of E. coli for 1 h, the biomass of E. coli decreased by about 13%, respectively (P < 0.05) (Supplemental Fig. S3). Based on the amount of ethanol produced by S. cerevisiae at four different time points, the corresponding concentration of ethanol solution was prepared to treat E. coli, and the change in biomass with ethanol concentration was determined. E. coli biomass decreased after ethanol treatment at different concentrations (Supplemental Fig. S4), indicating that ethanol had an obvious inhibitory effect on growth of E. coli.
Differences of growth of S. cerevisiae S288c wild-type, PFK1Δ, and TDH1Δ strains in co-culture with E. coli and expressions of key genes involved in ethanol synthesis
Growth of the PFK1Δ strain in the mixed culture was slower than that of S. cerevisiae S288c wild-type and TDH1Δ strains, and the PFK1Δ strain did not achieve higher biomass growth at the stationary stage (Fig. 4A). Meanwhile, the biomass of E. coli in co-culture with the PFK1Δ strain was the highest compared to that in co-culture with the other two strains (i.e., S. cerevisiae S288c wild-type and TDH1Δ strains) (Fig. 4B).
Growth of the Saccharomyces cerevisiae S288c wild-type, PFK1Δ, and TDH1Δ strains, and Escherichia coli co-cultured, respectively, and expression of key genes in ethanol synthesis after co-culture. A Biomass of the E. coli at different times after co-culture. B Biomass of the S288c wild-type, PFK1Δ, and TDH1Δ strains, at different times after co-culture. Differences in gene expression of ADH1, PDC1, PDC5, and PDC6 in co-culture of two strains and Escherichia coli at 8 h (C) and 16 h (D). *P < 0.05 compared to the S288c wild-type strain. **P < 0.01 compared to the S288c wild-type strain. ***P < 0.001 compared to the S288c wild-type strain
Based on the increase in mixed culture, 8 and 16 h were chosen as the time points for evaluating the expression of genes that play a crucial role in ethanol synthesis. After 8 h of mixed culture, the expression levels of PDC1, PDC5, and PDC6 in the PFK1Δ strain decreased by approximately 0.5-fold compared to the wild-type strain (P < 0.001) (Fig. 4C). But after 16 h of mixed culture, the expressions of ADH1, PDC1, and PDC5 in the PFK1Δ strain showed various degrees of upregulation (P < 0.01) (Fig. 4D). This showed that the presence of E. coli had a certain effect on ethanol synthesis in the PFK1Δ strain.
Discussion
S. cerevisiae, a commonly used model organism in industry, is prone to contamination by other microorganisms during fermentation (Nevoigt 2008), and interspecific competitiveness is crucial for the growth and survival of microorganisms (Hibbing et al. 2010). Out of the five strains of S. cerevisiae that had key genes involved in glucose metabolism knocked out to enhance its oxidation tolerance, the PFK1Δ strain displayed growth lag and reverse overshoot (Fig. 1A), and was frequently contaminated by other microorganisms. Previous studies have suggested that the utilization of glucose by PFK1 is critical (Yuan et al. 2020; Arvanitidis and Heinisch 1994), whereas the TDH1 gene is less critical for the use of glucose (Randez-Gil et al. 2020). The microbial growth can also be affected by nutrients, especially carbon sources (Olivares-Marin et al. 2018); there was no growth biomass anti-overshoot under the condition of a sufficient carbon source (Fig. 1E). Based on this, the lower growth of PFK1Δ in the lag and exponential growth stages (as shown in Fig. 1) indicates that the strain utilizes less glucose than the other two strains, which results in more residual glucose being available for PFK1Δ to use in the stationary phase, leading to higher growth. The data presented in Fig. 2 indicates that the glucose uptake and utilization efficiency of the PFK1Δ strain is lower than that of the other two strains, with respect to extracellular glucose residue content at different time points. The amount of residual sugar in the culture system could affect the growth of S. cerevisiae (Jansen et al. 2005; Nissen et al. 2003), so that more residual glucose remaining in the lag and exponential stages also provided the material base for the subsequent growth surpassing of the PFK1Δ strain in the stationary phase. Intracellular glucose at 20 h also confirmed the higher growth of the PFK1Δ strain than the other two strains in the stationary stage (Fig. 2E). Previous studies have demonstrated that the activity of the PFK1 gene can influence glycolysis flux, and this can subsequently impact the efficiency of glucose transport in cells (Papagianni and Avramidis 2011). The lower expression of HXT2, HXT5, and HXT6 in the PFK1Δ strain compared to that in the S. cerevisiae S288c wild-type strain (P < 0.05) (Fig. 3A) resulted in a lower glucose transport efficiency of in the PFK1Δ strain. Therefore, there was a greater extracellular glucose surplus in the PFK1Δ strain during the early phase (Fig. 2). Deletion of the key gene PFK1 in the glycolysis pathway (i.e., EMP, the Embden-Meyerhof-Parnas pathway) decreased the glucose utilization efficiency of S. cerevisiae, which in turn further affected its transport. Deletion of the key gene, PFK1 combined with decreased glucose transport efficiency, contributed to the lower growth rate of the PFK1Δ strain in the lag and exponential stages. However, on the other hand, microbes would survive as much as possible by adopting different strategies in extreme cases (Dumorné et al. 2017). Although the pentose phosphate pathway (HMP) was not the primary pathway for S. cerevisiae to consume carbon sources, such as glucose, when the Embden-Meyerhof-Parnas pathway was inhibited or blocked, HMP was activated as much as possible to ensure yeast survival (Li et al. 2012). Therefore, activation of the HMP pathway in the PFK1Δ strain was shown by an increase of ZWF1 gene expression (Fig. 3 B and D). The PFK1Δ strain consumed more residual glucose during the stationary phase at 20 h of culture compared to the forward growth phase, indicating a potential for reverse overshoot. The increased expression of HXT6 and HXT7 with high affinity for glucose (Fig. 3C) also indicated that the PFK1Δ strain continued to utilize glucose for growth in the later growth stage.
Ethanol is the main fermentation product of S. cerevisiae and glucose metabolism often affects ethanol synthesis (Sabater-Muñoz et al. 2020; Yang et al. 2022). The production of ethanol by the PFK1Δ strain was lower than that of the wild-type S288c and TDH1Δ strains of S. cerevisiae (as shown in Fig. 2 A and B), indicating poor growth during the exponential phase. Pyruvate is catalyzed by pyruvate decarboxylase (i.e., PDC) to produce acetaldehyde, which is further reduced by alcohol dehydrogenase (i.e., ADH) to form ethanol (Mithran et al. 2014). Although the expression level of PDC1 in S. cerevisiae is higher than that of PDC5 and PDC6 (Raj et al. 2014; Hohmann 1991), the catalytic efficiency of PDC5 is highest when the pyruvate amount is sufficient (Agarwal et al. 2013). In the early growth phase, the gene expression of PDC5 and PDC6 in the PFK1Δ strain decreased (P < 0.05) (Fig. 3E), indicating lower ethanol production efficiency in the PFK1Δ strain in the early growth stage. This result was also confirmed by the lower ethanol yield of the PFK1Δ strain during the early growth stage (Fig. 2 A and B). As the stationary phase was reached, despite an increase in PDC5 gene expression (Fig. 3F), the more crucial ADH1 gene expression decreased, hindering the ethanol synthesis efficiency in the PFK1Δ strain. On the other hand, the higher biomass of PFK1Δ at the stationary stage (Fig. 1A) was responsible for the similar ethanol yield of these two strains (Fig. 2D). The deletion of the PFK1 gene hindered the EMP pathway in the PFK1Δ strain, leading to a decrease in glucose uptake and utilization, which subsequently decreased ethanol production efficiency. The cell wall contact tests (Supplemental Fig. S1) and supernatant replacement (Supplemental Fig. S3) demonstrated that the growth of E. coli was impaired due to the exclusion of cell wall contact and other substances in the culture medium. The growth of E. coli was also inhibited by different concentrations of ethanol (Supplemental Fig. S4), and the ethanol content on the side inoculated with E. coli was higher than that on the uninoculated blank group (P < 0.01) (Supplemental Fig. S2B). The acetic acid secreted by E. coli, along with other detrimental substances to yeast cell growth, permeates into the yeast-containing MIC bottle through the filter membrane inducing the yeast cells to respond to this specific stimulus. The ethanol produced by S. cerevisiae showed directional flow and consistently moved towards the side exposed to external stimuli. These results further suggest the potential of ethanol as a defense weapon for S. cerevisiae. As a result, the decline in ethanol production efficiency caused by the PFK1 deletion diminishes the interspecies competitiveness of S. cerevisiae. The competitiveness of microorganisms can be evaluated by measuring their ability to grow in a competitive environment (Khonsari and Kollmann 2015). Therefore, the growth of S. cerevisiae and E. coli in mixed culture (Fig. 4 A and B) also suggests that PFK1Δ had the weakest interspecific competitiveness among the three strains in co-culture with E. coli.
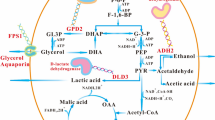
The expression of key genes in ethanol synthesis decreased in the early stage of the mixed culture (Fig. 4C), suggesting a lower ethanol production efficiency of PFK1Δ strain in the co-culture. The reduced ethanol yield resulted in the rapid growth of E. coli in the co-culture with the PFK1Δ strain. When microorganisms are subjected to more stress from environmental factors or other microorganisms, they exhibit certain defense behaviors (Guan et al. 2017); and with the extension of the time of feeling stress, the ability of microorganisms to respond to proactive or passive stress gradually increases. Accordingly, during the later stages of the mixed culture, the expression of genes critical to ethanol synthesis in the PFK1Δ strain increased (as shown in Fig. 4D), leading to an increase in ethanol synthesis, which in turn served to counteract the contaminating microorganisms. At this time (i.e., 16 h of co-culture), the biomass of E. coli in co-culture with the PFK1Δ strain reached the maximum, and the PFK1Δ strain enhanced its ability to produce ethanol as a defensive weapon to counter the enhanced threat from E. coli. However, in the early stage, the glucose uptake capacity and ethanol production capacity of PFK1Δ were weak; therefore, PFK1Δ was more susceptible to other microorganisms (i.e., E. coli here) than the wild-type strain and the other four knockout mutants (Fig. 5).
The deletion of PFK1, a crucial gene in the EMP pathway, can slow down the utilization of intracellular glucose and decrease the glucose uptake of yeast cells, leading to more residual glucose in the medium for the PFK1Δ strain to use for growth and reverse overshoot at the stationary stage. Meanwhile, the slower growth of the PFK1Δ strain in the early stage also reduced the yield of ethanol, which could be used as a defensive weapon to counter the competitiveness or thereat from E. coli, making the PFK1Δ strain less competitive than the wild-type strain while being co-cultured with other microorganisms (i.e., represented by E. coli here). The PFK1Δ strain’s decreased interspecies competitiveness led to increased biomass accumulation in the co-cultured E. coli, which in turn enhanced the PFK1Δ strain’s ethanol production efficiency and allowed it to counter the threat posed by the E. coli at the stationary stage. The results of this study could be valuable in understanding the regulation of the PFK1 gene on the growth and interspecies microbial competition behavior of S. cerevisiae. Moreover, this finding that regulating the expression of the PFK1 gene could enhance the growth ability, yield, and tolerance of S. cerevisiae has significant implications for the selection and optimization of strains in industrial fermentation. This could improve the efficiency and yield of the fermentation industry by adjusting the competitiveness of different yeast strains in the fermentation process. Additionally, regulating the expression level of PFK1 gene could also control the competition between S. cerevisiae and other microorganisms in food processing, improving the fermentation efficiency and taste and nutritional value of products. In future studies, the introduction of a target gene plasmid with an inducible promoter can be employed to achieve expression of the PFK1 gene over time periods. Such strategy could allow for rapid biomass accumulation and enhance interspecies competitiveness during the early stage, ultimately resulting in S. cerevisiae strains with heightened tolerance in the later stage. The findings of this study hold practical applications in various fields such as wine making, biofuel production, and other food or chemical production.
Data availability
All data generated or analyzed during this study are included and available in this published article (and its supplementary information files).
References
Agarwal PK, Uppada V, Noronha SB (2013) Comparison of pyruvate decarboxylases from Saccharomyces cerevisiae and Komagataella pastoris (Pichia pastoris). Appl Microbiol Biotechnol 97(21):9439–9449
Arroyo-López F, Salvadó Z, Tronchoni J, Guillamón JM, Barrio E, Querol A (2010) Susceptibility and resistance to ethanol in Saccharomyces strains isolated from wild and fermentative environments. Yeast 27:1005–1015
Arvanitidis A, Heinisch JJ (1994) Studies on the function of yeast phosphofructokinase subunits by in vitro mutagenesis. J Biol Chem 269(12):8911–8918
Buziol S, Warth L, Magario I, Freund A, Siemann-Herzberg M, Reuss M (2008) Dynamic response of the expression of hxt1, hxt5 and hxt7 transport proteins in Saccharomyces cerevisiae to perturbations in the extracellular glucose concentration. J Biotechnol 134(3–4):203–210
DangThu Q, Jang SH, Lee C (2020) Biochemical comparison of two glucose 6-phosphate dehydrogenase isozymes from a cold-adapted Pseudomonas mandelii. Extremophiles 24(4):501–509
Dong SJ, Lin XH, Li H (2015) Regulation of Lactobacillus plantarum contamination on the carbohydrate and energy related metabolisms of Saccharomyces cerevisiae during bioethanol fermentation. Int J Biochem Cell Biol 68:33–41
Dumorné K, Córdova DC, Astorga-Eló M, Renganathan P (2017) Extremozymes: a potential source for industrial applications. J Microbiol Biotechnol 27(4):649–659
Ghoul M, Mitri S (2016) The ecology and evolution of microbial competition. Trends Microbiol 24:833–845
Granato ET, Meiller-Legrand TA, Foster KR (2019) The evolution and ecology of bacterial warfare. Curr Biol 29(11):R521–R537
Guan N, Li J, Shin HD, Du G, Chen J, Liu L (2017) Microbial response to environmental stresses: from fundamental mechanisms to practical applications. Appl Microbiol Biotechnol 101(10):3991–4008
He XL, Liu B, Xu YL, Chen Z, Li H (2021) Effects of Lactobacillus plantarum on the ethanol tolerance of Saccharomyces cerevisiae. Appl Microbiol Biotechnol 105(6):2597–2611
Heinemann M, Sauer U (2010) Systems biology of microbial metabolism. Curr Opin Microbiol 13(3):337–343
Hibbing ME, Fuqua C, Parsek MR, Peterson SB (2010) Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol 8:15–25
Hohmann S (1991) PDC6, a weakly expressed pyruvate decarboxylase gene from yeast, is activated when fused spontaneously under the control of the PDC1 promoter. Curr Genet 20(5):373–378
Ingram LO (1990) Ethanol tolerance in bacteria. Crit Rev Biotechnol 9(4):305–319
Jansen MLA, Diderich JA, Mashego M, Hassane A, de Winde JH, Daran-Lapujade P, Pronk JT (2005) Prolonged selection in aerobic, glucose-limited chemostat cultures of Saccharomyces cerevisiae causes a partial loss of glycolytic capacity. Microbiology (reading) 151(Pt 5):1657–1669
Jin J, Nguyen T, Humayun S, Park S, Oh H, Lim S (2021) Characteristics of sourdough bread fermented with Pediococcus pentosaceus and Saccharomyces cerevisiae and its bio-preservative effect against Aspergillus flavus. Food Chem 345:128787
Kang X (2022) Regulation of glucose metabolism on oxidative stress tolerance of Saccharomyces cerevisiae. Beijing University of Chemical Technology, China
Kang X, Gao ZH, Zheng LJ, Zhang XR, Li H (2021) Regulation of Lactobacillus plantarum on the reactive oxygen species related metabolisms of Saccharomyces cerevisiae. LWT-Food Sci Technol 147:111492
Ke W, Chang S, Chen XJ, Luo SZ, Jiang ST, Yang PZ, Wu XF, Zheng Z (2015) Metabolic control analysis of L-lactate synthesis pathway in Rhizopus oryzae As 3.2686. Bioprocess Biosyst Eng 38(11):2189–99
Khonsari AS, Kollmann M (2015) Perception and regulatory principles of microbial growth control. PLoS One 10(5):e0126244
Kotte O, Zaugg JB, Heinemann M (2010) Bacterial adaptation through distributed sensing of metabolic fluxes. Mol Syst Biol 6:355
Lacerda MP, Oh EJ, Eckert C (2020) The model system Saccharomyces cerevisiae versus emerging non-model yeasts for the production of biofuels. Life (basel) 10:299
Li YJ, Tian CJ, Tian H, Zhang JL, He X, Ping WX, Lei H (2012) Improvement of bacterial cellulose production by manipulating the metabolic pathways in which ethanol and sodium citrate involved. Appl Microbiol Biotechnol 96(6):1479–1487
Liu B, Liu HQ, Zhang YX, Li H (2017) Control of Lactobacillus plantarum contamination in bioethanol fermentation by adding plantaricins. Int J Agri Biol 19:171–176
Madhavan A, Arun KB, Sindhu R, Krishnamoorthy J, Reshmy R, Sirohi R, Pugazhendi A, Awasthi MK, Szakacs G, Binod P (2021) Customized yeast cell factories for biopharmaceuticals: from cell engineering to process scale up. Microb Cell Fact 20:124
Mathew F, Goyal A (2023) Ethanol. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan
Mithran M, Paparelli E, Novi G, Perata P, Loreti E (2014) Analysis of the role of the pyruvate decarboxylase gene family in Arabidopsis thaliana under low-oxygen conditions. Plant Biol (stuttg) 16(1):28–34
Nevoigt E (2008) Progress in metabolic engineering of Saccharomyces cerevisiae. Microbiol Mol Biol Rev 72(3):379–412
Nissen P, Nielsen D, Arneborg N (2003) The relative glucose uptake abilities of non-Saccharomyces yeasts play a role in their coexistence with Saccharomyces cerevisiae in mixed cultures. Appl Microbiol Biotechnol 64(4):543–550
Olivares-Marin IK, González-Hernández JC, Regalado-Gonzalez C, Madrigal-Perez LA (2018) Saccharomyces cerevisiae exponential growth kinetics in batch culture to analyze respiratory and fermentative metabolism. J vis Exp 139:58192
Pandhal J, Noirel J (2014) Synthetic microbial ecosystems for biotechnology. Biotechnol Lett 36(6):1141–1151
Papagianni M, Avramidis N (2011) Lactococcus lactis as a cell factory: a twofold increase in phosphofructokinase activity results in a proportional increase in specific rates of glucose uptake and lactate formation. Enzyme Microb Technol 49(2):197–202
Park EY, Naruse K, Kato T (2012) One-pot bioethanol production from cellulose by co-culture of Acremonium cellulolyticus and Saccharomyces cerevisiae. Biotechnol Biofuels 5(1):64
Patra M, Salonen E, Terama E, Vattulainen I, Faller R, Lee BW, Holopainen J, Karttunen M (2006) Under the influence of alcohol: the effect of ethanol and methanol on lipid bilayers. Biophys J 90(4):1121–1135
Pina C, Santos C, Couto JA, Hogg T (2004) Ethanol tolerance of five non-Saccharomyces wine yeasts in comparison with a strain of Saccharomyces cerevisiae—influence of different culture conditions. Food Microbiol 21:439–447
Raj SB, Ramaswamy S, Plapp BV (2014) Yeast alcohol dehydrogenase structure and catalysis. Biochemistry 53(36):5791–5803
Randez-Gil F, Sánchez-Adriá IE, Estruch F, Prieto JA (2020) The formation of hybrid complexes between isoenzymes of glyceraldehyde-3-phosphate dehydrogenase regulates its aggregation state, the glycolytic activity and sphingolipid status in Saccharomyces cerevisiae. Microb Biotechnol 13(2):562–571
Sabater-Muñoz B, Mattenberger F, Fares MA, Toft C (2020) Transcriptional rewiring, adaptation, and the role of gene duplication in the metabolism of ethanol of Saccharomyces cerevisiae. mSystems 5(4):e00416-20
Sieuwerts S, Bron PA, Smid EJ (2018) Mutually stimulating interactions between lactic acid bacteria and Saccharomyces cerevisiae in sourdough fermentation. LWT-Food Sci Technol 90:201–206
Soden A, Francis IL, Oakey H, Henschke PA (2008) Effects of co-fermentation with Candida stellata and Saccharomyces cerevisiae on the aroma and composition of Chardonnay wine. Aust J Grape Wine R 6:21–30
Strommer J, Garabagi F (2008) ADH and PDC: Key roles for enzymes of alcoholic fermentation. In: Strommer J, Garabagi F (eds) Petunia: evolutionary, developmental and physiological genetics, 2nd edn. Springer, New York, pp 71–84
Strotz LC, Simões M, Girard MG, Breitkreuz L, Kimmig J, Lieberman BS (2018) Getting somewhere with the Red Queen: chasing a biologically modern definition of the hypothesis. Biol Lett 14(5):20170734
Westhoff S, Otto SB, Swinkels A, Bode B, van Wezel GP, Rozen DE (2020) Spatial structure increases the benefits of antibiotic production in Streptomyces. Evolution 74(1):179–187
Wu Q, Ling J, Xu Y (2014) Starter culture selection for making Chinese sesame-flavored liquor based on microbial metabolic activity in mixed-culture fermentation. Appl Environ Microbiol 80(14):4450–4459
Yang P, Jiang S, Lu S, Jiang S, Jiang S, Deng Y, Lu J, Wang H, Zhou Y (2022) Ethanol yield improvement in Saccharomyces cerevisiae GPD2 Delta FPS1 Delta ADH2 Delta DLD3 Delta mutant and molecular mechanism exploration based on the metabolic flux and transcriptomics approaches. Microb Cell Fact 21(1):160
Ye L, Berden JA, van Dam K, Kruckeberg AL (2001) Expression and activity of the Hxt7 high-affinity hexose transporter of Saccharomyces cerevisiae. Yeast 18(13):1257–1267
Yılmaz C, Gökmen V (2021) Formation of amino acid derivatives in white and red wines during fermentation: effects of non-Saccharomyces yeasts and Oenococcus oeni. Food Chem 343:128415
Yuan Y, Lin JY, Cui HJ, Zhao W, Zheng HL, Jiang ZW, Xiong XD, Xu S, Liu XG (2020) PCK1 deficiency shortens the replicative lifespan of Saccharomyces cerevisiae through upregulation of PFK1. Biomed Res Int 2020:3858465
Zengler K, Zaramela LS (2018) The social network of microorganisms-how auxotrophies shape complex communities. Nat Rev Microbiol 16(6):383–390
Funding
This work was supported by the National Nature Science Foundation of China (No. 82174531) and the Medicine and Health Science Technology Development Project of Shandong province, China (No. 202212060626).
Author information
Authors and Affiliations
Contributions
C. Z. and H. L. conceived and designed research. C. Z., S. H., and Y. Z. conducted the experiments. C. Z. and S. H. analyzed the data. C. Z., S. H., Y. Z., C. Y., and H. L. wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, C., Hou, S., Zhou, Y. et al. Regulation of the PFK1 gene on the interspecies microbial competition behavior of Saccharomyces cerevisiae. Appl Microbiol Biotechnol 108, 272 (2024). https://doi.org/10.1007/s00253-024-13091-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13091-9