Abstract
Transcription factor Cmr1 (Colletotrichum melanin regulation 1) and its homologs in several plant fungal pathogens are the regulators of the 1,8-dihydroxynaphthalene (DHN)-melanin biosynthesis pathway and have evolved functional diversification in morphology and pathogenicity. The fungal genus Alternaria comprises the group of “black fungi” that are rich in DHN-melanin in the primary cell wall and septa of the conidia. Some Alternaria species cause many economically important plant diseases worldwide. However, the evolution and function of Cmr1 homologs in Alternaria remain poorly understood. Here, we identified a total of forty-two Cmr1 homologs from forty-two Alternaria spp. and all contained one additional diverse fungal specific transcription factor motif. Phylogenetic analysis indicated the division of these homologs into five major clades and three branches. Dated phylogeny showed the A and D clades diverged latest and earliest, respectively. Molecular evolutionary analyses revealed that three amino acid sites of Cmr1 homologs in Alternaria were the targets of positive selection. Asmr1, the homolog of Cmr1 in the potato early blight pathogen, Alternaria solani was amplified and displayed the sequence conservation at the amino acid level in different A. solani isolates. Asmr1 was further confirmed to have the transcriptional activation activity and was upregulated during the early stage of potato infection. Deletion of asmr1 led to the decreased melanin content and pathogenicity, deformed conidial morphology, and responses to cell wall and fungicide stresses in A. solani. These results suggest positive selection and functional divergence have played a role in the evolution of Cmr1 homologs in Alternaria.
Key points
• Cmr1 homologs were under positive selection in Alternaria species
• Asmr1 is a functional transcription factor, involved in spore development, melanin biosynthesis, pathogenicity, and responses to cell wall and fungicide stresses in A. solani
• Cmr1 might be used as a potential taxonomic marker of the genus Alternaria
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanin, a natural amorphous polymer of high molecular weight, is a ubiquitous pigment found in microorganisms, plants, and animals (Cordero and Casadevall 2020). Many plant pathogenic ascomycete and dothideomycete fungi synthesize melanin following the 1,8-dihydroxynaphthalene (DHN) pathway (Kimura and Tsuge 1993; Tsuji et al. 2000; Eisenman and Casadevall 2012; Schumacher 2016; Wang et al. 2021b; Li et al. 2022). In fungi, melanin confers protection against abiotic stresses, such as ultraviolet radiation, extreme temperatures, reactive oxygen species (ROS), high osmotic pressure, hypertonic and heavy metal exposure, and dehydration (Cordero and Casadevall 2017). Melanin is also involved in fungal cell development of conidia, black appressoria, and sclerotia (Money et al. 1998; Kheder et al. 2012; Yu et al. 2015; Liang et al. 2018; Wang et al. 2021a; Zhou et al. 2022). In addition, DHN-melanin has a function in virulence of some phytopathogenic fungi during their interactions with the host immune response. For those fungi that produce melanized appressoria for host penetration, such as the rice blast pathogen Magnaporthe oryzae and the cucumber anthracnose pathogen Colletotrichum lagenarium, DHN-melanin is required for appressorial melanogenesis for pathogenicity (Kubo et al. 1982; Howard and Valent 1996). However, similarly, in some fungi like the gray mold pathogen Botrytis cinerea that do not form appressoria, DHN-melanin also correlates with their virulence. Mutants of B. cinerea defective in three melanin biosynthesis-related genes fail to produce melanin, but they show increased virulence (Zhang et al. 2015; Zhou et al. 2022).
Cmr1 (Colletotrichum melanin regulation 1) first described in Colletotrichum lagenarium, is an important transcription factor for regulating the expression of key enzymes in fungal DHN-melanin biosynthesis pathway, including polyketide synthase, 1,3,6,8-tetrahydroxynaphthalene reductase, scytalone dehydratase, 1,3,8-trihydroxynaphthalene reductase, and laccase (Tsuji et al. 2000; Eisenman and Casadevall 2012). Cmr1 is characterized by the presence of two types of DNA binding motifs, two Cys2His2 zinc finger motifs and one GAL4-like Zn(II)2Cys6 binuclear cluster DNA-binding motif. Until now, Cmr1 homologs that have been identified and confirmed their roles as the regulators in the DHN-melanin biosynthesis pathway of several plant fungal pathogens, include Pig1 in Magnaporthe grisea (Tsuji et al. 2000), Cmr1 in Cochliobolus heterostrophus (Eliahu et al. 2007), Bmr1 in Bipolaris oryzae (Kihara et al. 2008), Amr1 in Alternaria brassicicola (Cho et al. 2012), CmrA in Alternaria alternata (Fetzner et al. 2014), BcSMR1 in Botrytis cinerea (Zhou et al. 2017), Zmr1 in Zymoseptoria tritici (Krishnan et al. 2018), CgCmr1 in Colletotrichum gloeosporioides (Wang et al. 2021a, b), and StMR1 in Setosphaeria turcica (Zhang et al. 2022). Apart from a role in controlling DHN-melanin biosynthesis, Cmr1 homologs also affect the morphology and pathogenicity of some phytopathogenic fungi, suggesting the functional diversification of Cmr1 homologs. It is noteworthy that some Cmr1 homologs including BcSMR1, Zmr1, and CgCmr1 have no role in pathogenicity, while StMR1 positively regulates the pathogenicity and Amr1 negatively affects the pathogenicity. Further investigating the function of other Cmr1 homologs will provide support about the functional difference in pathogenicity of Cmr1 homologs.
The worldwide-distributed fungal genus Alternaria is the typical representative of Dematiaceae and comprises the group of “black fungi”, which are rich in DHN-melanin in the primary cell wall and septa of their conidia (Carzaniga et al. 2002; Thomma 2003). It is currently classified into 24 sections based on phylogenetic and morphological studies (Woudenberg et al. 2013). Some Alternaria species are fungal pathogens of many economically important plant diseases, which result in huge losses in agricultural production and postharvest agricultural products throughout the world (Thomma 2003). A. brassicicola is responsible for black spot disease on almost all cultivated Brassica species including cabbage, broccoli, canola, mustard, and rapeseed in the world (Neergaard 1945; Sigareva and Earle 1999; Westman et al. 1999). A. alternata is a pathogen on over 100 plant hosts including cereal crops, vegetables, and fruits (Rotem 1994). For example, A. alternata causes Alternaria brown spot of citrus (Gai et al. 2021), leaf blight on Ophiopogon japonicus (Wang et al. 2021a, b), and black spot of kiwifruit (Huang et al. 2021). Alternaria solani is the causal agent of early blight, one of the most destructive diseases of potato and tomato worldwide (Agrios 2005). In China, early blight ranks number 1 fungal disease on potato (Ma et al. 2020). A. solani also causes early blight on other members of the Solanaceae family including pepper, eggplant, black nightshade, horse nettle, and Jerusalem cherry (Farr and Rossman 2023). Although potato early blight is a devastating disease, only a few factors involved in pathogenicity have been identified in A. solani, such as toxins: alternaric acid and solanapyrones A, B, and C (Brian et al. 1949).
Currently, two Cmr1 homologs including Amr1 in A. brassicicola (Cho et al. 2012) and CmrA in A. alternata (Fetzner et al. 2014) have been identified and characterized in Alternaria. The function of Cmr1 homologs in regulating the DHN-melanin biosynthesis is highly conserved in A. brassicicola, A. alternata, and other fungi described above (Tsuji et al. 2000; Eliahu et al. 2007; Kihara et al. 2008; Cho et al. 2012; Fetzner et al. 2014; Zhou et al. 2017; Krishnan et al. 2018; Wang et al. 2021a, b; Zhang et al. 2022). Interestingly, based on the amino acid sequence comparison between Amr1 and CmrA, there was one amino acid insertion at site 934 in Amr1 and 31 polymorphic sites (data not shown), suggesting that the amino acid sequences have diverged in Amr1 and CmrA. Furthermore, Amr1 negatively regulates the pathogenicity of A. brassicicola (Cho et al. 2012). Cmr1 homologs in phytopathogenic fungi have evolved to differentiate in pathogenicity, which may relate to the interaction with their host plants. As a consequence, given the availability of the genome sequences of 44 Alternaria species to date and our laboratory stored 10 A. solani isolates from different geographic locations in China, we raised the following questions. How did Cmr1 homologs evolve in Alternaria and in A. solani? What kind of functions the Cmr1 homolog in A. solani may have? Of particular interest, whether there is functional difference in pathogenicity regulated by Cmr1 homologs in Alternaria? Here, we exploited Alternaria genomes for identifying Cmr1 homologs followed by investigating the diversity and evolutionary relationship of Cmr1 homologs in Alternaria as well as those in A. solani. We also engineered one A. solani isolate deficient in the production of a Cmr1 homolog using a gene knockout technology to test whether Cmr1 homolog acts as a virulence factor and whether DHN-melanin is important for pathogenesis of A. solani.
Materials and methods
Plants, fungal isolates, and growth conditions
Potato (Solanum tuberosum cv. Zaodabai and cv. Favorita) plants were grown and maintained in a growth chamber (23 °C day/15 °C night temperatures with 14 h of light). A. solani isolates (Supplemental Table S1) and mutants were maintained as glycerol stocks. For conidial sporulation, the wild type (WT) A. solani isolate SH0806 and its mutants were grown on potato carrot agar (PCA) medium at 25 °C.
Database mining
The protein sequence of Amr1 was used as a query to BlastP searching against all annotated protein databases of Alternaria spp. available in the websites of GenBank, National Center for Biotechnology Information (http://www.ncbi.nih.gov), JGI, Joint Genome Institute, US Department of Energy (http://www.jgi.doe.gov), and AGD, Alternaria genome database (http://alternaria.vbi.vt.edu) to identify Cmr1 homologs in Alternaria. For some Alternaria species without genome annotation, first, their corresponding genome sequences were predicted into genes through the AUGUSTUS server (Stanke and Morgenstern 2005). Second, the local protein databases were constructed using the Blast-2.12.0 program (Altschul et al. 1997). Third, BLASTP searching was performed against the generated local protein databases using Amr1 as a query. Finally, all achieved protein sequences were searching against the PFAM database in SMART server (Letunic et al. 2020) to confirm the existence of characteristic motifs of Cmr1.
Sequence analyses
The amino acid sequences corresponding to individual motifs of Cmr1 homologs were aligned using the ClustalX program (Thompson et al. 1997) and submitted to WebLogo server (Crooks et al. 2004) to calculate the consensus sequences. MEGA7.0.21 (Kumar et al. 2016) was used to make multiple sequence alignments of Cmr1 homologs followed by reconstructing a phylogenetic tree using the maximum likelihood (ML) method with 1000 bootstrapping replications. The Cmr1 (GenBank accession number ABI81496) in C. heterostrophus was used as an outgroup. The ML tree was used to estimate divergence times using the method of Reltime implemented in MEGA7.0.21. Two calibration points were used. That is, the node between Cmr1 in C. lagenarium and Pig1 in M. grisea was constrained to 299 million years ago (MYA) following the estimated divergence time (355 MYA) of Colletotrichum and Alternaria. Their divergence time estimates were derived from the TimeTree server (Kumar et al. 2022).
The selection pressures were performed using the CODEML program within the Phylogenetic Analysis by Maximum Likelihood (PAML) software package with the ML method (Yang 1997; Nielsen and Yang 1998; Yang et al. 2000; Yang and Bielawski 2000). A series of likelihood ratio tests (LRTs) were carried out to test for positive selection by comparing the null models (M0, M1a, M7) with their corresponding alternative models (M3, M2a, M8). The empirical Bayes theorem implemented in CODEML was used to calculate the posterior probability that a particular amino acid site belongs to a given selection class (neutral, deleterious or advantageous) (Yang 1997). Amino acid sites with a high probability coming from advantageous class of sites are more likely to be under positive selection.
The protein sequences of polyketide synthase, PksA, two α-hydrolases, AygA and AygB, scytalone dehydratase Brm1, two 1,3,6,8-tetrahydroxynaphthalen (T4HN) reductases, Brm2 and Brm3, and seven laccases, LccA, LccB, LccC, LccD, LccE, LccF, and LccG from A. alternata (JGI Protein IDs 111952, 115293, 105009, 105968, 111954, 112254, 112523, 114657, 115320, 114031, 111569, 110245, and 116332, respectively) (Gao et al. 2022) were used to search against the A. solani BMP0185 genome for identifying for corresponding orthologus genes. Using CmrA (UniProt accession number A0A075QC79) as a template, the structures of Asmr1 and Amr1 were predicted by SWISS-MODEL server (Waterhouse et al. 2018), visualized, and aligned by PyMOL (Schrödinger and DeLano, http://www.pymol.org/pymol).
DNA manipulations, gene cloning, and DNA sequencing
Genomic DNA was extracted from mycelia using the fungal genomic DNA extraction kit (Solarbio, Beijing, China). Based on the sequence of the Cmr1 homolog (AGD accession number ASL_PT00349) in A. solani, a pair of oligonucleotide primers Asmr1F/Asmr1R was designed for the amplification of the entire coding region of asmr1 from A. solani isolates. The amplified fragment was cloned into pTOPO-Blunt vector (Aidlab, Hong Kong, China) and confirmed by DNA sequencing. All primers used in this paper were listed in Supplemental Table S2.
Transcriptional activation assay
The gene fragment amplified from pTOPO:Asmr1 (carrying the sequence ON962808) using the primers Asmr1-BD-EcoRIF/Asmr1-BD-SalIR and a EcoRI, SalI-linearized bait vector pGBKT7 (Clontech, Mountain View, CA, USA) were homologously recombined using the ClonExpress® MultiS One Step Cloning Kit (Vazyme, Nanjing, China) to obtain the construct pGBKT7:Asmr1 (intron). To delete the intron sequences, four gene fragments were amplified from pGBKT7:Asmr1 (intron) using the primers Asmr1-BD-EcoRIF/Asmr1-IN1-firstR, Asmr1-IN1-secondF/Asmr1-IN2-firstR, Asmr1-IN2-secondF/Asmr1-IN3-firstR, and Asmr1-IN3-secondF/Asmr1-BD-SalIR, respectively. They were homologously recombined with a EcoRI, SalI-linearized pGBKT7 vector to achieve the construct pGBKT7:Asmr1, which was confirmed by DNA sequencing. The transcriptional activation assay was performed following the instructions of the Yeast One-hybrid Kit (Clontech, Mountain View, CA, USA). Preparation and transformation of Y2H Gold competent cells were carried out following the instructions of the Yeast Transformation Kit (Coolaber, Beijing, China). The nutrient deficient medium was purchased from Coolaber (Beijing, China).
RNA manipulations and quantitative real-time RT-PCR
Total RNA extraction from infected leaves of potato 0, 2, 4, 6, 8, 10, 12, 24, 36, 48, 72, and 96 h post-inoculation (hpi) with A. solani SH0806 was carried out using the FastePure® Plant Total RNA Isolation Kit (Vazyme, Nanjing, China). cDNA was synthesized using the ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China). The primers were designed to anneal to sequences in regions spanning at least one intron to ensure no genomic DNA disturbance except those for aygB, brm3, and lccG due to the lack of introns. Real-time detection was carried out on the CFX ConnectTM Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) and real-time PCR data was analyzed using the program GENEX (Bio-Rad, Hercules, CA, USA). The relative expression levels of asmr1 and thirteen genes including pksA, aygA, aygB, brm1, brm2, brm3, lccA, lccB, lccC, lccD, lccE, lccF, and lccG [JGI (https://mycocosm.jgi.doe.gov/Altso1/Altso1.home.html) with transcript IDs 115231, 121832, 121729, 112195, 115171, 120096, 117354, 116243, 119329, 111647, 1181013, 115914, and 114865, respectively] were standardized to those of A. solani actin1 (GenBank accession number MK388241.1) and ef1α (GenBank accession number MN370901.1), respectively.
Generation of the asmr 1 deletion construct
The deletion construct was made by replacement of the gene with a hygromycin B (HygB) resistance cassette. The HygB phosphotransferase gene (hph) was used as a selective marker. The gene replacement construct was produced by a double-joint PCR method (Yu et al. 2004) and consisted of PCR fragments of 5′ and 3′ flanking region sequences of asmr1 overlapping within hph. The 5′ and 3′ flanking regions of asmr1 were amplified from A. solani using Asmr1-5F/Asmr1-5R and Asmr1-3F/Asmr1-3R, respectively. The HygB resistance cassette was amplified from pAg1-H3 (Zhang et al. 2003) with hph1349F/hph1349R. The primer set Asmr1-nestF/Asmr1-nestR was used to amplify the fused knockout construct, which was confirmed by DNA sequencing.
Generation of the complemented construct of the Δasmr1-2
The Δasmr1-2 mutant was complemented with the wild-type (WT) allele under its native promoter. The gene fragment containing a 1497 bp 5′ flanking region and the coding region of asmr1 without the stop codon was amplified from isolate SH0806 using the primer set Asmr1-CF/Asmr1-CR and verified by DNA sequencing. The confirmed gene fragment and the linearized pYF11-gfp-Gen vector (Chen et al. 2018) were co-transformed into yeast strain XK1-25 using the Yeast Transformation Kit (Coolaber, Beijing, China) to achieve the fusion construct through the yeast gap repair approach. The resulting construct, pYF11:Asmr1 was verified by DNA sequencing.
Preparation of fungal protoplasts
Preparation of fungal protoplasts was conducted as previously described with a minor modification (Akamatsu et al. 1997). Details are as follows: Agar plugs containing WT A. solani (SH0806) or its mutants were cultured on potato dextrose agar (PDA) until spore sporulation. Spores were collected, transferred into potato dextrose broth (PDB), and shaken at 120 rpm at 28 °C for 1–2 days. Mycelia were harvested using miracloth (EMD Biosciences, Rockland, MA, USA), rinsed with sterilized ddH2O three times, and re-suspended in 50 ml of filter sterilized solution of enzymes containing 10 mg/ml lysing enzymes (Sigma, St. Louis, MO, USA) and 10 mg/ml Kitalase (Wako Pure Chemical Industries, Ltd., Osaka, Japan), which were dissolved in 0.7 M NaCl. The suspension was shaken at 70 rpm at 30 °C for 3–4 h, filtered by miracloth, and centrifuged at 3000 × g for 5 min. After the removal of the supernatant, the pellets of protoplasts were gently re-suspended with 0.7 M NaCl and centrifuged at 3000 × g for 5 min. Protoplasts were re-suspended with 1 ml of STC solution [1.2 M sorbitol, 10 mM Tris–HCl (pH 7.5), 10 mM CaCl2] and centrifuged at 3000 × g for 5 min. Pelleted protoplasts were re-suspended with an appropriate volume of STC solution to obtain a final solution containing 1 × 108 protoplasts. The protoplasts were checked under the light microscope and the number of protoplasts was determined with a hemacytometer.
Transformation of A. solani and molecular confirmation of transformants
A. solani protoplasts were transformed following the method of Akamatsu et al. (1997) with some modification. One to 5 µg plasmid DNA and 5 µl of heparin sodium (5 mg/ml) were mixed with 1 ml of STC solution containing 1 × 108 protoplasts and incubated on ice for 30 min. One third amount of SPTC solution (STC solution containing 40% PEG 4000) was added into the mixture and incubated at room temperature for 20 min. Subsequently, 50 ml of RM (regeneration medium) (0.1% yeast extract, 0.1% casein enzymatic hydrolysate, 1 M sucrose) was added into the transformation mixture and incubated at 25 °C overnight followed by plating on PDA containing 100 µg/ml Hyg B. After incubating at 28 °C for 7–14 days, putative transformants were selected under two rounds of single-spore isolation. To obtain stable hph expressing putative transformants, single-spore isolated putative transformants were sub-cultured on PDA without HygB and PDA with HygB in turn for five generations. Selected transformants were verified by PCR with five primer pairs, H850F/H852R to check the presence of hph; Asmr1-InF/Asmr1-InR to check the presence of asmr1; Asmr1-nestF/Asmr1-nestR to check whether asmr1 was replaced by hph; Asmr1-nestF/H855R to check the correct integration of HygB resistance cassette into the upstream region of asmr1; H856F/nestR to check the correct integration of HygB resistance cassette into the downstream region of asmr1. Southern blot hybridization according to the manufacturer’s instructions of the DIG-High Prime DNA Labeling and Detection Kit II (Roche, Indianapolis, IN, USA) followed by DNA sequencing using the primer pair HphCF/HphCR was performed to confirm the presence of one single copy of hph and the deletion of asmr1 in PCR verified transformants. Integration of the WT asmr1 allele under its native promoter into the Δasmr1-2 mutant genome was confirmed with PCR using the asmr1 internal region primer pair Asmr1-InF/Asmr1-InR.
Examination of colony growth and conidia
WT A. solani and its mutants from glycerol stocks were inoculated on PDA in the dark at 25 °C for 5 days. Agar plugs (5 mm in diameter) containing fresh mycelia on PDA were sub-cultured on PDA, PCA, or potato agar (PA) and PDA containing 0.2 g/L Congo red, 0.02% SDS (sodium dodecyl sulfate), 10 mM H2O2, 5 ppm mancozeb, 5 ppm chlorothalonil, 5 ppm azoxystrobin, 1 M KCl, 1 M NaCl, 1 M sorbitol, 10 mM FeSO4, 1.5 mM CuSO4 or 250 mM CaCl2 and incubated at 25 °C for 5 days. The diameter of each colony was measured using a Vernier caliper and the inhibitory rate of fungal mycelial growth was calculated. All experiments were conducted three times. Three replicates were used for each experiment. Significant differences in colony diameter and inhibitory rate were determined by one-way analysis of variance (ANOVA). Conidia were collected from PCA with sterilized ddH2O and examined for their morphology using a Nikon TE2000 inverted fluorescence microscope (Nikon, Miato, Japan).
Extraction and detection of fungal melanin content
Extraction of fungal melanin was conducted following the method described by Yang et al. (2012) with modification. A quarter of 1 g of fresh mycelia was collected, transferred into 10 ml of 1 M NaOH solution, incubated at 100 °C water bath for 5 h, and filtered using one layer of filter paper. The filtrate was adjusted to pH to 2.0 using HCl, incubated at 4 °C overnight, and centrifuged at 6000 × g for 15 min. The pellets collecting melanin were dissolved in 5 ml of 1 M NaOH and the absorbance of the solution at 459 nm was measured by UV spectrophotometer. The melanin content (Y, mg/g) of fungal mycelia was calculated as the following formula: Y = (X + 0.0114)/0.0495, where Y represents the yield of fungal melanin and X indicates the absorbance value at 459 nm. Three replicates were used for the experiment. Significant differences in melanin yield were determined by ANOVA.
Measurement of ROS
WT A. solani and its mutants from glycerol stocks were inoculated on PDA in the dark at 25 °C for 5 days. Agar plugs (5 mm in diameter) containing fresh mycelia were transferred to PDA and incubated at 25 °C for 3 days. Three days-old fungal cultures were stained with nitroblue tetrazolium (NBT) reagent (Beyotime Biotechnology, Shanghai, China) to detect the production of ROS.
Plant infection assays
A total of 20 µl of suspension containing 1 × 105 spores/ml from WT A. solani isolate SH0806 or its mutants was used to inoculate the detached leaves of potato. The inoculated leaves were placed in a plastic tray lined with wet paper towels, tightly fitted with a transparent plastic cover, and kept in a growth chamber at 25 °C under a photoperiod of 12 h. For quantitative real-time RT-PCR, leaves were harvested at 12 time points as described above, individually frozen in liquid nitrogen, and stored at –80 °C prior to RNA extraction. For pathogenicity test, the symptoms and necrotic lesion size were recorded at 14 days post-inoculation (dpi). The necrotic lesion size was calculated based on the square area (cm2) of the lesion. The experiment was performed three times. Each time had three technical repeats. Significant differences in lesion size were determined by ANOVA.
Results
Cmr1 homologs in Alternaria all contain one additional diverse fungal specific transcription factor motif
To identify Cmr1 homologs in the genus Alternaria, we mined publicly available Alternaria genomes representing 44 Alternaria species (Supplemental Table S3). After verification of the motifs, a total of 44 Cmr1 homologs (one per each species) including previously reported CmrA in A. alternata and Amr1 in A. brassicicola were identified (Supplemental Table S4 and Supplemental Figs. S1 and S2). Cmr1 homologs from Alternaria carthami and Alternaria destruens were excluded from further analysis due to the presence of gaps within their coding regions. Excluding three introns including two located in 5′ non-coding region (75/97/98/99 bp and 75/76/83/84/85 bp in length) and one in 3′ non-coding region (55/56/59 bp in length), all remaining 42 Cmr1 homologs ranged from 3027 to 3033 bp in length corresponding to 1009 to 1011 predicted amino acids (Supplemental Table S4). All 42 Cmr1 homologs contained the expected motifs located at N-terminus: two Cys2His2 zinc finger motifs (ZnF_C2H2, SM000355) at amino acid positions 2 to 24 and 30 to 52, respectively, as well as one GAL4-like Zn(II)2Cys6 binuclear cluster DNA-binding motif (GAL4, SM00066) at amino acid positions 73 to 116 (Fig. 1a). In addition, they all carried one fungal specific transcription factor motif (fungal_trans, PF04082) at the C-terminus (amino acid positions 498 to 740 or 499 to 741) (Fig. 1a). Weblogo analysis of motifs of Cmr1 homologs revealed that the amino acid sequences within each of two ZnF_C2H2 motifs and the GAL4 motif were completely conserved (Fig. 1b–d). In contrast, the amino acid sequences within the fungal_trans motif were diverse at 17 sites in Cmr1 homologs (Fig. 1e). Our data indicates that Cmr1 homologs in Alternaria contain one extra diverse fungal_trans motif except for three fully conserved characteristic motifs of Cmr1.
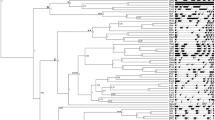
Structure and phylogeny of Cmr1 homologs in Alternaria. a Motifs of Cmr1 homologs in Alternaria are schematically shown. The motifs were predicted by SMART server at http://smart.embl-heidelberg.de. ZnF_C2H2 represents the Cys2His2 zinc finger motif (SM000355). GAL4 indicates the GAL4-like Zn(II)2Cys6 binuclear cluster DNA-binding motif (SM00066). Fungal_trans denotes the fungal specific transcription factor motif (PF04082). The numbers above each motif describes the amino acid locations of the motif at the full length protein. b Consensus sequence in the first ZnF_C2H2 motif, c in the second ZnF_C2H2 motif, d in the GAL4 motif, and e in the fungal_trans motif were estimated based on multiple sequence alignment of 42 Cmr1 homologs using WebLogo server at http://www.weblogo.berkeley.edu. The diverse amino acid sites are indicated by asterisks in red. The bigger the letter is, the more conserved the amino acid site is. f ML tree based on the predicted protein sequences of 42 Cmr1 homologs was constructed using MEGA7.0.21 and rooted with Cmr1 in C. heterostrophus. Confidence values higher than 70% shown at the nodes were obtained from 1000 bootstrap replications. The A, B, C, D, and E clades are shaded by gray
Five clades of Cmr1 homologs emerge in Alternaria
To determine the evolutionary relationship of Cmr1 homologs in Alternaria, a phylogeny (phylogenetic tree) was generated based on alignment of their amino acid sequences using the ML method implemented in the program MEGA7.0.21 (Kumar et al. 2016). Forty-two Cmr1 homologs were classified into five closely related genetic groups, clades A, B, C, D, and E supported with high bootstrap values of 95, 99, 99, 75, and 88, respectively, and three individual branches (Fig. 1f). The clade A comprised 13 species including members of the Alternaria section Alternata and 2 species belonging to an unclassified Alternaria section, Alternaria alstroemeriae and Alternaria fragariae. The B clade was composed of 8 species, which were included in the Alternaria section Porri, such as A. solani. The C clade consisted of Alternaria atra and Alternaria consortialis, belonging to the Alternaria section Ulocladioides. The D clade also contained 2 species, A. brasssicicola from the Alternaria section Brassicicola and Alternaria brassicae from an unclassified Alternaria section. The E clade included 12 species, all from the Alternaria section Infectoriae. The results showed that the A, B, C, and D clades were more closely related to each other than to the E clade. In addition, the A clade was the most divergent one. Three species including Alternaria panax, Alternaria gansuensis, and Alternaria rosae formed individually three branches. Note that, phylogenetic analysis placed CmrA in A. alternata, the Cmr1 homolog in A. solani, and Amr1 in A. brassicicola in A, B, and D distinct clades, respectively. When coupled with the divergence in host range of A. alternata, A. solani, and A. brassicicola, it appears that these Cmr1 homologs have been under selection for functional divergence during the interaction with their hosts or living environments. Most surprising, the phylogeny of Cmr1 homologs followed the authoritative classification system of the genus Alternaria established by Woudenberg et al. (2013). It implies that Cmr1 can be used as a potential taxonomic marker of the genus Alternaria, which can distinguish at least five groups of Alternaria species.
The A and D clades diverge latest and earliest, respectively
To investigate how divergent Cmr1 homologs appeared in Alternaria, we estimated the divergence times for all branching points in the phylogeny of 42 Cmr1 homologs using the Reltime ML method implemented in the Timetree Wizard window of the program MEGA7.0.21 (Kumar et al. 2016) and two calibration points (see “Materials and methods” for details). As seen in Fig. 2, the last common ancestor of group I and group II of the 42 Cmr1 homologs had an estimated age of 135.77 MYA (million years ago). The origin of group I including the A, B, C, and D clades as well as two individual branches, A. panax and A. gansuensis, was estimated to be 97.14 MYA. The origin of group II was inferred to be 18.28 MYA, suggesting that group II emerged later than group I. In addition, among the five clades, the D clade diverged earliest (59.93 MYA), followed by the B clade (18.5 MYA), the E clade (11.2 MYA), and the C clade (10.79 MYA), and the A clade diverged latest (8.71 MYA).
Dated phylogeny of Cmr1 homologs in Alternaria. The topology was based on ML analysis of the amino acid sequences of 42 Cmr1 homologs. The Cmr1 in C. heterostrophus was used as an outgroup. Molecular-clock estimates of median divergence times using the Reltime ML method implemented in MEGA7.0.21 and two calibration points (see “Materials and methods” for details) are given on each node in MYA (million years ago). The scale is in MYA
Cmr1 homologs in Alternaria are under positive selection
The value of the ratios of nonsynonymous to synonymous nucleotide substitution rates in protein-coding DNA sequences (ω = dN/dS) is the indicator of the type of selection pressures (Yang and Bielawski 2000). The ω values of 1, < 1, and > 1 represent neutral, positive, and negative selection, respectively. To examine the selection pressure underlying sequence diversification in Cmr1 homologs in Alternaria, we analyzed the 42 Cmr1 homologs using the ML method (a statistical distribution to model ω variations among sites along the protein) implemented in the program CODEML in the PAML software package (Yang 1997). First, LRTs were performed to test for selection. In details, three pairs of ML models of codon substitution such as M3/M0, M8/M7, and M2a/M1a were used (Yang et al. 2000) (Table 1). Both the discrete model M3 and the selection model M2a did not identify any amino acid sites under positive selection. In contrast, the model M8 suggested that 0.2% of sites were under positive selection with ω = 1.659. The LRT for comparing M8 with M7 was 2△L = 2 × [–13151.053–(–13,156.009)] = 9.912, which is greater than the χ2 critical value (9.21 at the 1% significance level, with degrees of freedom (df) = 2) (Table 1). Thus, the null model M7 which does not allow for the presence of positive selection sites with ω > 1 was rejected, indicating that the detected positive selection under the discrete M8 was statistically significant at P value < 0.05. Second, the amino acid sites under positive selection when LRT tested the presence of sites with ω > 1 were identified. This was achieved by using the empirical Bayes theorem to calculate the (posterior) probability. We identified three sites including 171 S, 927 G, and 966 S implicated as being under positive selection with the confidence level of 98%, 78.6%, and 69.5%, respectively, under model M8. Interestingly, none of the three identified positive selection sites were located in any of the four identified protein motifs of Cmr1 homologs (Fig. 1a and Supplemental Fig. S3). The results suggest that positive selection has affected the evolution of Cmr1 homologs in Alternaria.
The Cmr1 homolog is fully conserved at the amino acid level in A. solani isolates
To study whether there is sequence polymorphism in Cmr1 homologs in A. solani, we amplified, cloned, and sequenced Cmr1 homologs from ten A. solani isolates from different potato or tomato producing areas in China including the northeast (Suihua, Keshan, Daqing, Jiagedaqi District, and Benxi), the northwest (Dingxi), the north (Bejing and Shandong), and the south (Jiansu) (Supplemental Table S1). Multiple alignments of the nucleotide sequences of these ten Cmr1 homologs and two additional Cmr1 homologs from isolates BMP 0185 and altNL03003 (The Netherlands) revealed that polymorphisms were detected at 10 of 3277 nucleotides (data not shown). All polymorphic sites were located in the exon sequences," however, they could not cause amino acid changes. The results imply that Cmr1 homologs at amino acid levels are completely conserved and appear to undergo negative selection in A. solani isolates from different geographic locations.
Asmr1 exhibits transcriptional activity in yeast
To link the sequence analysis and the function of the Cmr1 homolog in A. solani, we validated the transcription factor activity of the Cmr1 homolog in A. solani using the transcription activation assay. The construct pGBKT7 carrying the open reading frame of Asmr1 (we designated the Cmr1 homolog amplified from isolate SH0806 A. solani melanin regulation transcription factor 1 or Asmr1) was first achieved. After transforming into the yeast strain Y2H GOLD, both empty pGBKT7 and pGBKT7:Asmr1 constructs enabled Y2H GOLD to grow on selective dropout medium without tryptophan (SD-T). However, only the strain Y2H GOLD carrying pGBKT7:Asmr1 construct could grow on SD medium without tryptophan, histidine, and adenine (SD-T-H-A). In addition, transformed colonies of pGBKT7:Asmr1 exhibited a blue color on SD-T-H-A medium supplemented with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-α-Gal) (Fig. 3a). The results indicate that Asmr1 is able to activate the transcription of reporter genes including His3, Ade2, and Mel1, supporting that Asmr1 is a functional transcription factor.
Transcriptional activation analysis of Asmr1 in yeast and verification of asmr1 deletion and complementation mutants. a Yeast Y2H GOLD strains carrying the pGBKT7 vector (negative control) and pGBKT7:Asmr1 construct grew on SD-T and SD-T-H-A + X-α-Gal medium. b Schematic diagram of replacement of the asmr1 coding region with a HygB resistance cassette. The lowest panel shows the sequencing results of the Δasmr1-2 mutant with the primer pair, HphF/HphR. c Southern blot analysis of the asmr1 deletion mutants with the hph probe amplified with the primer set Hph1349F/Hph1349R and the asmr1 probe amplified with the primer pair Asmr1-InF/Asmr1-InR. Abbreviations: WT = wild-type A. solani; Δasmr1-1, Δasmr1-2, Δasmr1-3, asmr1 deletion mutants; Δasmr1-2C, complementation mutant Δasmr1-2:asmr1
Asmr1 is involved in spore development and melanin biosynthesis
To further investigate the function of Asmr1, a deletion mutant of the asmr1 gene was constructed by replacing the coding region with a HygB resistance cassette (Fig. 3b). Three HygB resistant transformants, namely, Δasmr1-1, Δasmr1-2, and Δasmr1-3 were obtained. The results of PCR analysis followed by Southern hybridization and DNA sequencing showed that Δasmr1-1 was an ectopic insertion mutant, whereas Δasmr1-2 and Δasmr1-3 were correct asmr1 replacement mutants (Supplemental Fig. S4a, Fig. 3b, c). We complemented the Δasmr1-2 mutant with the WT allele. PCR and RT-PCR analyses confirmed that Δasmr1-2C was a correctly asmr1 complemented transformant of mutant Δasmr1-2 (Supplemental Fig. S4b).
We determined the effect of asmr1-deficiency on conidia in A. solani. The WT and the Δasmr1-2 and Δasmr1-2C mutants all produced conidia on PCA and showed little difference in conidia production (data not shown). However, the conidia of the Δasmr1-2 mutant were pale, with non-black color, and deformed, in comparison with the dark brown to black color and intact conidia of the WT and the Δasmr1-2C mutant (Fig. 4a and Supplemental Fig. S5). In certain individual conidia of the Δasmr1-2 mutant, some part was colorless or white while another part looked brownish. It is worth noting that the beak of the conidia of the Δasmr1-2 mutant became shorter compared with the WT and the Δasmr1-2C mutant (Fig. 4a and Supplemental Fig. S5). We subsequently measured the melanin content in A. solani. The color of the crude melanin extract from the Δasmr1-2 mutant looked very light and was close to transparent. In contrast, that from the WT and the Δasmr1-2C mutant displayed brown or dark brown color (Fig. 4b, c). Compared to WT, the melanin yield of the Δasmr1-2 mutant significantly decreased (Fig. 4c). Consequently, we quantified the transcript levels of A. solani orthologs of 13 DHN-melanin biosynthesis-related genes in fungi. Compared to WT, ten genes including pksA, aygA, aygB, brm1, brm2, brm3, lccB, lccC, lccE, and lccF were all significantly less expressed in the Δasmr1-2 mutant, whereas the three laccase genes lccA, lccD, and lccG were all significantly upregulated in the Δasmr1-2 mutant (Fig. 4d), suggesting that the expression of the former genes was positively dependent on Asmr1 and that of the latter negatively controlled by Asmr1. Altogether, the results indicate that Asmr1 is involved in conidial morphogenesis and the regulation of melanin biosynthesis in A. solani.
Effect of asmr1 deletion on conidial morphology and melanin production in A. solani. a Comparison of conidial morphology between mutants and wild-type on PCA. b Crude extracts of melanin of wild-type and its mutants. c Precipitation (left panel) and yield (right panel) of crude extracts of melanin of wild-type and its mutants. The melanin content (Y, mg/g) of fungal mycelia was calculated as the following formula: Y = (X + 0.0114)/0.0495, where Y represents the yield of fungal melanin, and X indicates the absorbance value at 459 nm. d Expression levels of A. solani orthologs of genes involved in DHN-melanin biosynthesis in wild-type and the Δasmr1-2 mutant and normalized to ef1α expression levels. Error bars present standard deviations of three biological replicates. The significant difference in yield of melanin and relative expression was determined by one-way analysis of variance (ANOVA). **P < 0.01; ***P < 0.001 (comparison with wild-type). Abbreviations: WT, wild-type A. solani; Δasmr1-2, asmr1 deletion mutant; Δasmr1-2C, complementation mutant Δasmr1-2:asmr1
Decreased pathogenicity of the Δasmr1-2 mutant in potato
To test whether the Asmr1 is involved in the infection process of A. solani in potato, we determined the expression of asmr1 during infection using quantitative real-time RT-PCR. The asmr1 gene showed elevated levels of expression throughout early infection stage from 0 to 12 hpi (Fig. 5a), suggesting that Asmr1 may involve in the infection process of A. solani in potato. This finding promoted us to perform a pathogenicity test. Two weeks after inoculation, the necrotic lesions caused by the Δasmr1-2 mutant were significantly smaller than those caused by the WT (Fig. 5b, c). The results suggest that the pathogenicity of the Δasmr1-2 mutant decreases in potato.
Time course expression of asmr1 during infection of potato by A. solani and pathogenicity assay of the asmr1 deletion mutant on potato. a Expression levels of asmr1 at 0, 2, 4, 6, 8, 10, 12, 24, 36, 48, 72, and 96 hpi of susceptible potato cv. Zaodabai infection with A. solani isolate SH0806 and normalized to A. solani actin1 expression levels. Error bars present standard deviations of three biological replicates. b Inoculated leaves of susceptible potato cv. Favorita with wild-type, asmr1 deletion mutant, and complementation mutant. c The area size (cm.2) of necrotic lesion caused by inoculation at 14 dpi. Values represent means from three independent experiments with three replicates each. The significant difference in lesion size was determined by ANOVA. ***P < 0.001 (comparison with wild-type). Abbreviations: WT, wild-type A. solani; Δasmr1-2, asmr1 deletion mutant; Δasmr1-2C, complementation mutant Δasmr1-2:asmr1
Responses of the Δasmr1-2 mutant to cell wall and fungicide stresses
Melanin plays a role in protecting fungi against a diverse array of stresses (Cordero and Casadevall 2017). To this end, we examined the effect of stressors including cell wall inhibitors, oxygen stressor, fungicides, osmotic stressors, and metal ions on the Δasmr1-2 mutant. The mycelial growth of the Δasmr1-2 mutant was significantly inhibited in the presence of Congo red or SDS (Fig. 6a), suggesting that Asmr1 may be involved in the regulation of the cell-wall-related pathway in A. solani. Similarly, the mycelial growth of the Δasmr1-2 mutant was also significantly reduced on the medium containing 10 mM H2O2 (Fig. 6a). This is likely due to the increase of ROS in the Δasmr1-2 mutant, which results in a counterbalance between ROS and H2O2. Thus, we decided to examine the ROS content in the Δasmr1-2 mutant through staining 3-day-old fungal cultures with NBT reagent. NBT reacts with superoxide anion to form blue or blue purple formazan. The higher the content of superoxide anion, the darker the color (Halliwell and Gutteridge 1985). As seen in Fig. 6b, the entire colony and hyphae of the Δasmr1-2 mutant exhibited a blue purple color, whereas both WT and the Δasmr1-2C mutant changed not in color. The results indicate that the ROS content in the Δasmr1-2 mutant is much higher than that of WT. Perhaps, without protection from melanin, asmr1-deficient A. solani increases the ROS content to combat against external adverse environmental conditions.
Effects of asmr1 deletion on A. solani responses to cell wall and oxygen stressors and determination of ROS contents. a Comparison of mycelial growth inhibitory rate between mutants and wild-type on PDA in the presence of indicated chemicals. Values represent means from three independent experiments with three replicates each. The significant difference in growth inhibitory rate was determined by ANOVA. *P < 0.05; ***P < 0.001 (comparison with wild-type). b Colony (upper panel) and hyphae (lower panel) of 3-day-old WT, asmr1 deletion mutant, and complementation mutant on PDA stained with NBT reagent. Abbreviations: WT, wild-type A. solani; Δasmr1-2, asmr1 deletion mutant; Δasmr1-2C, complementation mutant Δasmr1-2:asmr1
Three economic and practical fungicides including mancozeb, chlorothalonil, and azoxystrobin for the prevention and control of potato early blight were used in this study. Compared to WT, the Δasmr1-2 mutant showed a significant decrease in the mycelial growth on the medium supplemented with 5 ppm of mancozeb, chlorothalonil, or azoxystrobin (Fig. 7). These results indicate that the deletion of asmr1 results in the reduced tolerance of A. solani to these three fungicides. It also indirectly suggests that the effect of mancozeb on preventing and controlling potato early blight is better than that of chlorothalonil and azoxystrobin.
Effects of the asmr1 deletion on A. solani responses to three fungicides. Comparison of mycelial growth inhibitory rates between mutants and wild-type on PDA in the presence of indicated fungicides. Values represent means from three independent experiments with three replicates each. Significant differences in growth inhibitory rates were determined by ANOVA. ***P < 0.001 (comparison with wild-type). Abbreviations: WT, wild-type A. solani; Δasmr1-2, asmr1 deletion mutant; Δasmr1-2C, complementation mutant Δasmr1-2:asmr1
The mycelial growth of the Δasmr1-2 mutant was comparable to that of the WT and Δasmr1-2C mutant on the medium containing KCl, NaCl or sorbitol (Supplemental Fig. S6a). Since KCl, NaCl, and sorbitol are related to fungal osmotic stress, we speculate that Asmr1 may not be involved in the regulation of the osmotic stress pathway in A. solani. In addition, the mycelial growth of the WT and of the Δasmr1-2 and Δasmr1-2C mutants were completely inhibited on the medium supplemented with Fe2+ (Supplemental Fig. S6b). The colony size was not significantly different between the WT and the Δasmr1-2 mutant under Cu2+ stress. However, the Δasmr1-2 mutant showed a significant reduction in the mycelial growth on the medium containing Ca2+. Altogether, it suggests that Asmr1 may be involved in the regulation of the A. solani response to Ca2+, but not to Fe2+ and Cu2+.
We also determined the effect of asmr1-deficiency on vegetative growth of the WT and the Δasmr1-2, and Δasmr1-2C mutants on PDA, PCA, or PA (Supplemental Fig. S7). The Δasmr1-2 mutant displayed orange or near pink and light orange color (with less dense aerial hyphae) on PDA and PCA, respectively, compared to the black-colored WT. On PA lacking sugar, WT changed from black on the medium containing sugar to gray, while the Δasmr1-2 mutant remained little changed. Compared to the WT, the colony size in the Δasmr1-2 mutant was significantly smaller on PDA, PCA, or PA. The phenotype was comparable for both the Δasmr1-2C mutant and the WT. The results suggest that Asmr1 affects the vegetative growth in A. solani.
Both Asmr1 and Amr1 share highly similar structures with CmrA
Through multiple sequence alignment of Asmr1, Amr1, and CmrA, a total of 39 polymorphic amino acid sites were identified (data not shown). Therefore, we predicted the protein structures of Asmr1 and Amr1 through the SWISS-MODEL server (Waterhouse et al. 2018) using the public available protein structure of CmrA as a template. As seen in Fig. 8, both Asmr1 and Amr1 folded into a structure highly similar to CmrA. The alpha-helix, beta-sheet, and random coil were accounted for 34.4%, 3%, and 53.1% of the entire protein structure, respectively. Six and two out of 39 polymorphic sites were located in an alpha-helix or a beta-sheet while the remaining 31 sites were in random coils. Furthermore, two of six polymorphic sites in alpha-helixes were located in the fungal_trans motif. Taken together, the results suggest that structural conservation occurs in Asmr1, Amr1, and CmrA.
The structures of CmrA, Asmr1, and Amr1. The protein structures of Asmr1 and Amr1 were predicted by SWISS-MODEL server at http://swissmodel.expasy.org using CmrA (UniProt accession number A0A075QC79) as a template. The structures were visualized and aligned using the PyMOL software
Discussion
In this study, we found that Cmr1 homologs were present in all examined Alternaria species and defined their gene structures with regard to consensus sequences at three intron junctions. This will eventually contribute to future prediction, identification, and annotation of Cmr1 homologs in other Alternaria species. Furthermore, identification of three completely conserved motifs suggests that the functional conservation of these motifs occurs in Cmr1 homologs. Most notably, one extra diverse fungal_trans motif was identified in Cmr1 homologs in Alternaria. However, until now, not all identified Cmr1s and their homologs in other fungi carry this motif. The motif is present in Cmr1 of C. heterostrophus, Bmr1, and StMR1, but not in Cmr1 of C. lagenaria, Pig1, BcSMR1, Zmr1, and CgCmr1 (Supplemental Fig. S8). It is not clear whether the fungal_trans motif originally existed in Cmr1 or was obtained during evolution. The phylogeny of eleven described Cmr1 homologs including those from other fungi and Asmr1, Amr1, and CmrA revealed that the six Cmr1 homologs with fungal_trans motif from six different fungal species belonging to four distinct genera clustered together to form one clade (Supplemental Fig. S8b). Given the fungal_trans motif sequences were polymorphic, therefore, we compared the motif structures of the six Cmr1 homologs. All fungal_trans motifs of the six Cmr1 homologs folded into a similar structure (Supplemental Fig. S9a, b, c). Despite the different morphologies of these six species, as well as the taxonomic status of four different genera, Cmr1 homologs still share similarities among the six species, such as the close phylogenetic relationship, the similar gene structure, that all carry the four identified protein motifs, and similar folded structures of the fungal_trans motif. We speculate that Cmr1 homologs may have undergone divergent evolution in different fungi. This is likely due to the long period of selection pressure from their ecological niches and hosts.
Due to the large differences in the morphology, identification of Alternaria spp. is usually not correct only based on one single gene. However, the reliability in Alternaria spp. identification using four to five genes is high (Woudenberg et al. 2013). We found here that Cmr1 could distinguish different groups of Alternaria spp., indicating that Cmr1 has potential application values in taxonomy. It therefore not only augments the known function of melanin, but also expands the identification methods of Alternaria spp., that is, Cmr1 might be used as a novel taxonomic marker, just like the secondary metabolite toxin (Andersen et al. 2008; Patriarca et al. 2019).
Here, we show that positive selection has promoted divergence of Cmr1 homologs in Alternaria at three amino acid sites. These three positive selection sites were found to be located in random coils, which may alter the secondary structure of Cmr1. To rapidly adapt to novel and changing environments for increasing the survival probability, biological populations need to maintain new beneficial mutations or a standing genetic variation of adaptive traits (Messer and Petrov 2013). Melanization is such an adaptive trait. It is known that transcription factors that regulate expression of effectors in plant-pathogen interactions, secreted enzymes, and secondary metabolites, are required for the adaption of plant pathogenic fungi to the host environment (van der Does and Rep 2017). Thus, adaptive changes in the coding region of Cmr1 homologs in Alternaria might be responsible for the regulation of the variation in melanin accumulation. It would allow fungal populations to adapt and specialize on new hosts, or to adapt/tolerate to stress conditions. Future functional analyses, such as site-directed mutagenesis and identification of host targets of Cmr1 could be explored to help understanding the nature of adaptive changes in Cmr1 homologs in Alternaria.
Asmr1 was confirmed to have transcriptional activation activity and be involved in the melanin biosynthesis pathway. Noticeably, the deletion of asmr1 did not completely abolish melanin production, suggesting the presence of a crosstalk between melanin biosynthesis and other signaling pathways regulating melanin. The MAP kinase (MAPK) pathways are critical for fungi to regulate responses to environmental stimuli (Zhang et al. 2021). Three conserved MAPK pathways including the cell wall integrity (CWI), invasive growth (IG), and high osmolarity growth (HOG) pathways have been well characterized in plant fungal pathogens (Zhang et al. 2021). Indeed, one IG pathway gene, AbSte7 in A. brassicicola and one CWI pathway gene, AaSLT2 in A. alternata are required for melanin biosynthesis (Yago et al. 2021; Xu et al. 2016). In addition, AaSet2 encoding a histone methyltransferase is involved in melanin accumulation (Meng et al. 2022). Histone methylation is known to be vital for transcriptional regulation and diverse biological processes. We predict that the incomplete decrease of melanin accumulation in the Δasmr1-2 mutant could be due to the regulation role from other melanin-related signaling pathways, such as the MAPK pathway.
Asmr1 also affected other biological processes of A. solani. After the deletion of asmr1, the structure of certain conidia of A. solani became abnormal and the numbers of transverse and longitudinal septa of conidia also changed. This finding is similar to results presented in previous reports about the involvement of DHN-melanin in regulating longitudinal septal number and cell wall integrity of A. alternata conidia (Yago et al. 2021; Kheder et al. 2012) and that of CmrA in controlling the spore morphology and spore formation of A. alternata (Fetzner et al. 2014). However, the deletion of amr1 in A. brassicicola has no effect on the conidial morphology (Cho et al. 2012). It suggests that the functional divergence of spore morphology occurs in Cmr1 homologs in Alternaria. In addition, we found that there were some pigments remaining in conidia, although the melanized degree of conidia and hyphae decreased after the deletion of asmr1. A recent study reported that many proteins including the CmrA transcription factor, which are required for the DHN-melanin biosynthesis pathway, are also involved in fungal toxin production via the perylene quinones biosynthesis pathway in A. alternata (Gao et al. 2022). Therefore, we speculate that Asmr1 may be a global transcription factor participating in multiple pathways.
Except for controlling melanin biosynthesis and morphogenesis, Asmr1 was required for the resistance to Congo red or SDS. It is critical for plant fungal pathogens to maintain the cell wall integrity and stability for disease establishment within the host (Zhang et al. 2021). Melanin is located within the fungal cell wall (Thomma 2003). The absence of Asmr1 might affect the cell wall integrity and stability of A. solani, further influencing the tolerance of A. solani to these cell wall stressors. Plant pathogenic fungi secrete a series of cell wall-degrading enzymes (CWDEs) during infection (Kubicek et al. 2014). Some researches indicate that the type and activity of fungal CWDEs is associated with metal ions, such as Ca2+, Zn2+, and Mg2+ etc. (Piero and Pascholati 2000; Martino et al. 2000; Sucheta et al. 2001). For instance, Ca2+ inhibits the activity of polygalacturonase in Fusarium oxysporum f. sp. vasinfectum infecting cotton (Piero and Pascholati 2000). One possible explanation for the observed phenotype of the decreased tolerance to Ca2+ in the Δasmr1-2 mutant could be that the deletion of asmr1 might affect the cell wall integrity of A. solani, leading to a change of cell wall permeability, facilitating the easier attack of certain CWDEs by Ca2+. Therefore, this would influence the tolerance of A. solani to Ca2+.
Similar to the silenced mutant of CmrA in A. alternata (Fetzner et al. 2014), the Δasmr1-2 mutant of A. solani reduced tolerance to H2O2. In contrast, a deletion mutant of Amr1 in A. brassicicola remained unchanged in the sensitivity against H2O2 (Cho et al. 2012). This implies the diversification of responding to oxidative stress in Cmr1 homologs in Alternaria. Plants generate toxic ROS, primarily H2O2 and superoxide as a defense response against pathogens. For a plant fungal pathogen, its sensitivity to ROS likely depends on the effectiveness of its own ROS detoxification ability. In A. alternata, the detoxification of ROS is regulated by the redox-responsive transcription regulators YAP1 and SKN7 or by a nonribosomal peptide synthetase (NPS) mediated pathway (Lin et al. 2009; Yang et al. 2009; Chen et al. 2012, 2013). The ability of A. alternata to detoxify ROS is also required for virulence on citrus (Lin et al. 2009; Yang et al. 2009). Hence, the lack of Asmr1 might likely affect the ability of A. solani to effectively detoxify H2O2 through impairing ROS detoxification pathway, further influencing the tolerance of A. solani to external H2O2 stress.
One of the interesting findings of this study is the observation on the involvement of a melanin-regulating gene in fungicide sensitivity. Asmr1 was found to be required for cellular resistance to mancozeb, chlorothalonil, and azoxystrobin. Mancozeb is shown to be an inhibitor of metal- and/or sulfhydryl-containing enzymes related to lipid metabolism, respiration, and ATP production, resulting in the inhibition of fungal spore germination (Dias et al. 2010; Gullino et al. 2010). Chlorothalonil has a multi-site mode of action that inhibits specific NAD thiol-dependent glycolytic and respiratory enzymes (Tillman et al. 1973). Azoxystrobin is a quinone outside inhibitor (QoI) fungicide with a site-specific mode of action. It inhibits mitochondrial respiration by binding to Qo site of the cytochrome b, blocking electron transfer, and thus halting ATP synthesis (Bartlett et al. 2002). Based on the mode of action of these tested fungicides, we predict that the reduced level of melanin due to the deletion of asmr1 might lead to the decreased detoxification ability of A. solani on substances affecting fungal spore germination (mancozeb/chlorothalonil), glycolysis (chlorothalonil), and respiration (azoxystrobin/chlorothalonil). Genome-wide expression analysis between the Δasmr1-2 mutant and the WT under appropriate conditions are worthy to do to further characterize the function of Asmr1, and to understand its role in abiotic stress response.
Asmr1 was found to be related to the pathogenicity in A. solani. Given the involvement of the Δasmr1-2 mutant in the sensitivity to cell wall stressors, it seems likely that the ability of the Δasmr1-2 mutant to respond to cell wall stress was associated with its decreased pathogenicity. Asmr1 might regulate the expression of a set of genes encoding CWDEs that are important for decomposing and utilizing plant material during infection. In this context, it is worth noting that many glycoside hydrolases 61 family genes are highly expressed in the deletion mutant of Amr1 in A. brassicicola during pathogenesis (Cho et al 2012). Thus, A. brassicicola can quickly and efficiently utilize pectin and other cell wall components of green cabbage plant cells, causing the host cells to be more easily damaged. Another explanation for the decreased pathogenicity may be a connection between melanin and spore germination or the ROS detoxification ability. Although the deletion of asmr1 did not affect the yield of conidia, the deformed conidia in the Δasmr1-2 mutant might influence spore germination. The possibility of a positive relationship between Asmr1 and CWDEs/spore germination/ROS detoxification could be explored further through experimental investigation of expression profiles between the Δasmr1-2 mutant and WT during the infection on potato using the RNA-seq approach followed by functional assays.
While in the same genus, Amr1 can negatively regulate the pathogenicity in A. brassicicola (Cho et al. 2012). Asmr1 positively regulated the pathogenicity in A. solani. It suggests there is the pathogenicity diversification regulated by Cmr1 homologs in Alternaria. Significantly, their predicted secondary structures were almost identical, despite the sequence variation between Asmr1, Amr1, and CmrA. This is possibly due to the fact that the amino acid substitutions among them include a high proportion of the same physico-chemical groups, such as nonpolar and non-charged amino acids. Considering the conservation of the protein structure and diversification in pathogenicity, we speculate that the structural architecture is under high selective constraint in the function of Asmr1 and Amr1, which is driven by the co-evolution between pathogens A. solani and A. brassicicola and their corresponding hosts. With the increase in the genomic sequences available of Alternaria spp., this will contribute to improve our understanding the evolutionary relationships among Cmr1 homologs in Alternaria. This study provides an experimental framework to identify Cmr1 homologs in Alternaria spp. and explore their evolution and function. In combination with the identification of targets of Cmr1 homologs, this will ultimately help to make a link between the evolution of Cmr1 homologs and their functional roles in the biology of Alternaria spp.
Data availability
The sequences of Cmr1 homologs from A. solani isolates have been deposited in GenBank under accession numbers ON962808 (isolate SH0806) and OP302825 to OP302833 (isolates DX0917, DQ0803, JG0805, KS0809, BX0917, BJPO2207, BJTO2207, JSTO2207, and SDTO2207, respectively).
References
Agrios GN (2005) Plant pathology, 5th edn. Elsevier Academic Press, London, pp 453–456
Akamatsu H, Itoh Y, Kodama M, Otani H, Kohmoto K (1997) AAL-toxin deficient mutants of Alternaria alternata tomato pathotype by restriction enzyme mediated integration. Phytopathol 87(9):967–972. https://doi.org/10.1094/PHYTO.1997.87.9.967
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search program. Nucleic Acids Res 25(17):3389–3402. https://doi.org/10.1093/nar/25.17.3389
Andersen B, Dongo A, Pryor BM (2008) Secondary metabolites profiling of Alternaria dauci, A. porri, A. solani, and A. tomatophila. Mycol Res 112(Pt 2):241–250. https://doi.org/10.1016/j.mycres.2007.09.004
Bartlett DW, Clough JM, Godwin JR, Hall AA, Hamer M, Parr-Dobrzanski B (2002) The strobilurin fungicides. Pest Manag Sci 58(7):649–662. https://doi.org/10.1002/ps.520
Brian PW, Curtis PJ, Hemming HG, Unwin CH, Wright JM (1949) Alternaric acid, a biologically active metabolic product of the fungus Alternaria solani. Nature 164(4169):534. https://doi.org/10.1038/164534a0
Carzaniga R, Fiocco D, Bowyer P, O’Connell RJ (2002) Localization of melanin in conidia of Alternaria alternata using phage display antibodies. Mol Plant Microbe Interact 15(3):216–224. https://doi.org/10.1094/MPMI.2002.15.3.216
Chen LH, Lin CH, Chung KR (2012) Roles for SKN7 response regulator in stress resistance, conidiation and virulence in the citrus pathogen Alternaria alternata. Fungal Genet Biol 49(10):802–813. https://doi.org/10.1016/j.fgb.2012.07.006
Chen LH, Lin CH, Chung KR (2013) A nonribosomal peptide synthetase mediates siderophore production and virulence in the citrus fungal pathogen Alternaria alternata. Mol Plant Pathol 14(5):497–505. https://doi.org/10.1111/mpp.12021
Chen Y, Zheng SY, Ju ZZ, Zhang CQ, Tang GF, Wang J, Wen ZY, Chen W, Ma ZH (2018) Contribution of peroxisomal docking machinery to mycotoxin biosynthesis, pathogenicity and pexophagy in the plant pathogenic fungus Fusarium graminearum. Environ Microbiol 20(9):3224–3245. https://doi.org/10.1111/1462-2920.14291
Cho Y, Srivastava A, Ohm RA, Lawrence CB, Wang KH, Grigoriev IV, Marahatta SP (2012) Transcription factor Amr1 induces melanin biosynthesis and suppresses virulence in Alternaria brassicicola. PLoS Pathog 8(10):e1002974. https://doi.org/10.1371/journal.ppat.1002974
Cordero RJB, Casadevall A (2017) Functions of fungal melanin beyond virulence. Fungal Biol Rev 31(2):99–112. https://doi.org/10.1016/j.fbr.2016.12.003
Cordero RB, Casadevall A (2020) Melanin. Curr Biol 30(4):R142–R143. https://doi.org/10.1016/j.cub.2019.12.042
Crooks GE, Hon G, Chandonia JM, Brenner SE (2004) WebLogo: a sequence logo generator. Genome Res 14:1188–1190. https://doi.org/10.1101/gr.849004
Dias PJ, Teixeira MC, Telo JP, Sá-Correia I (2010) Insights into the mechanisms of toxicity and tolerance to the agricultural fungicide mancozeb in yeast, as suggested by a chemogenomic approach. OMICS 14(2):211–227. https://doi.org/10.1089/omi.2009.0134
Eisenman HC, Casadevall A (2012) Synthesis and assembly of fungal melanin. Appl Microbiol Biotechnol 93:931–940. https://doi.org/10.1007/s00253-011-3777-2
Eliahu N, Igbaria A, Rose MS, Horwitz BA, Lev S (2007) Melanin biosynthesis in the maize pathogen Cochliobolus heterostrophus depends on two mitogen-activated protein kinases, Chk1 and Mps1, and the transcription factor Cmr1. Eukaryot Cell 6(3):421–429. https://doi.org/10.1128/EC.00264-06
Farr DF, Rossman AY (2023) Fungal databases, U.S. national fungus collections, ARS, USDA, retrieved 26 June 2023 from https://nt.ars-grin.gov/fungaldatabases
Fetzner R, Seither K, Wenderoth M, Herr A, Fischer R (2014) Alternaria alternata transcription factor CmrA controls melanization and spore development. Microbiology 160:1845–1854. https://doi.org/10.1099/mic.0.079046-0
Gai YP, Ma HJ, Chen YN, Li L, Cao YZ, Wang MS, Sun XP, Jiao C, Riely BK, Li HY (2021) Chromosome-scale genome sequence of Alternaria alternata causing Alternaria brown spot of citrus. Mol Plant Microbe Interact 34(7):726–732. https://doi.org/10.1094/MPMI-10-20-0278-SC
Gao J, Wenderoth M, Doppler M, Schuhmacher R, Marko D, Fischer R (2022) Fungal melanin biosynthesis pathway as source for fungal toxins. mBio 13(3):e0021922. https://doi.org/10.1128/mbio.00219-22
Gullino ML, Tinivella F, Garibaldi A, Kemmitt GM, Bacci L, Sheppard B (2010) Mancozeb: past, present, and future. Plant Dis 94(9):1076–1087. https://doi.org/10.1094/PDIS-94-9-1076
Halliwell B, Gutteridge J (1985) Free radicals in biology and medicine. Clarendon Press, Oxford
Howard RJ, Valent B (1996) Breaking and entering: host penetration by the fungal rice blast pathogen Magnaporthe grisea. Annu Rev Microbiol 50:491–512. https://doi.org/10.1146/annurev.micro.50.1.491
Huang K, Tang JM, Zou Y, Sun XC, Lan JB, Wang W, Xu PP, Wu XW, Ma R, Wang Q, Wang ZS, Liu J (2021) Whole genome sequence of Alternaria alternata, the causal agent of black spot of kiwifruit. Front Microbiol 12:713462. https://doi.org/10.3389/fmicb.2021.713462
Kheder AA, Akagi Y, Akamatsu H, Yanaga K, Maekawa N, Otani H, Tsuge T, Kodama M (2012) Functional analysis of the melanin biosynthesis genes ALM1 and ByRM2-1 in the tomato pathotype of Alternaria alternata. J Gen Plant Pathol 78(1):30–38
Kihara J, Moriwaki A, Tanaka N, Tanaka C, Ueno M, Arase S (2008) Characterization of the BMR1 gene encoding a transcription factor for melanin biosynthesis genes in the phytopathogenic fungus Bipolaris oryzae. FEMS Microbiol Lett 281(2):221–227. https://doi.org/10.1111/j.1574-6968.2008.01101.x
Kimura N, Tsuge T (1993) Gene cluster involved in melanin biosynthesis of the filamentous fungus Alternaria alternata. J Bacteriol 175:4427–4435. https://doi.org/10.1128/jb.175.14.4427-4435
Krishnan P, Meile L, Plissonneau C, Ma X, Hartmann FE, Croll D, McDonald BA, Sánchez-Vallet A (2018) Transposable element insertions shape gene regulation and melanin production in a fungal pathogen of wheat. BMC Biol 16(1):78. https://doi.org/10.1186/s12915-018-0543-2
Kubicek CP, Starr TL, Glass NL (2014) Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu Rev Phytopathol 2:427–451. https://doi.org/10.1146/annurev-phyto-102313-045831
Kubo Y, Suzuki K, Furusawa I, Yamamoto M (1982) Effect of tricyclazole on appressorial pigmentation and penetration from appressoria of Colletotrichum lagenarium. Phytopathology 72:1198–1200. https://doi.org/10.1094/Phyto-72-1198
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Kumar S, Suleski M, Craig JM, Kasprowicz AE, Sanderford M, Li M, Stecher G, Hedges SB (2022) TimeTree5: an expanded resource for species divergence times. Mol Biol Evol 39(8):msac174. https://doi.org/10.1093/molbev/msac174
Letunic I, Khedkar S, Bork P (2020) SMART: recent updates, new developments and status in 2000. Nucleic Acids Res 49(D1):D458–D460. https://doi.org/10.1093/nar/gkaa937
Li H, Wang D, Zhang DD, Geng Q, Li JJ, Sheng RC, Xue HS, Zhu H, Kong ZQ, Dai XF, Klosterman SJ, Subbarao KV, Chen FM, Chen JY (2022) A polyketide synthase from Verticillium dahliae modulates melanin biosynthesis and hyphal growth to promote virulence. BMC Biol 20:125. https://doi.org/10.1186/s12915-022-01330-2
Liang Y, Xiong W, Steinkellner S, Feng J (2018) Deficiency of the melanin biosynthesis genes SCD1 747 and THR1 affects sclerotial development and vegetative growth, but not pathogenicity in Sclerotinia sclerotiorum. Mol Plant Pathol 19(6):1444–1453. https://doi.org/10.1111/mpp.12627
Lin CH, Yang SL, Chuang KR (2009) The YAP1 homolog-mediated oxidative stress tolerance is crucial for pathogenicity of the necrotrophic fungus Alternaria alternata in citrus. Mol Plant Microbe Interact 22(8):942–952. https://doi.org/10.1094/MPMI-22-8-0942
Ma ZZ, Ren BY, Zhao ZH, Li CG, Shen J, Yan S (2020) Comparative analysis of the occurrence and control of pests and diseases in four major potato producing areas in China. J Plant Protect (Chinese) 47(3):463–470
Martino E, Coisson JD, Lacourt I, Favaron F, Bonfante P, Perotto S (2000) Influence of heavy metals on production and activity of pectinolytic enzymes in ericoid mycorrhizal fungi. Mycol Res 104(7):825–833. https://doi.org/10.1017/S0953756299002099
Meng S, Huang S, Liu J, Gai Y, Li M, Duan S, Zhang S, Sun X, Yang Q, Wang Y, Xu K, Ma H (2022) Histone methylation is required for virulence, conidiation, and multi-stress resistance of Alternaria alternata. Front Microbiol 13:924476. https://doi.org/10.3389/fmicb.2022.924476
Messer PW, Petrov DA (2013) Population genomics of rapid adaptation by soft selective sweeps. Trends Ecol Evol 28(11):659–669. https://doi.org/10.1016/j.tree.2013.08.003
Money NP, Caesar-TonThat TC, Frederick B, Henson JM (1998) Melanin synthesis is associated with changes in hyphopodial turgor, permeability, and wall rigidity in Gaeumannomyces graminis var. graminis. Fungal Genet Biol 24(1–2):240–251. https://doi.org/10.1006/fgbi.1998.1052
Neergaard P (1945) Danish species of Alternaria and Stemphylium. Oxford University Press, London, United Kingdom
Nielsen R, Yang ZH (1998) Likelihood models for detecting positively amino acid sites and applications to the HIV-1 envelope gene. Genetics 148:929–936. https://doi.org/10.1093/genetics/148.3.929
Patriarca A, Cabral LDC, Pavicich MA, Nielsen KF, Andersen B (2019) Secondary metabolite profiles of small-spored Alternaria support the new phylogenetic organization of the genus. Int J Food Micr 291:135–143. https://doi.org/10.1016/j.ijfoodmicro.2018.11.022
Piero RM, Pascholati SF (2000) Cellulase production by Fusarium oxysporum f. sp. vasinfectum and the role in pathogenicity on cotton plants. Summa Phytopathologica 26(3):336–341
Rotem J (1994) The genus Alternaria: biology, epidemiology and pathogenicity. APS Press, St. Paul, Minn, USA
Schumacher J (2016) DHN melanin biosynthesis in the plant pathogenic fungus Botrytis cinerea is based on two developmentally regulated key enzyme (PKS)-encoding genes. Mol Microbiol 99(4):729–748. https://doi.org/10.1111/mmi.13262
Sigareva MA, Earle ED (1999) Camalexin induction in intertribal somatic hybrids between Camelina sativa and rapid-cycling Brassica oleracea. Theor Appl Genet 98:164–170. https://doi.org/10.1007/s001220051053
Stanke M, Morgenstern B (2005) AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res 33:W465-467. https://doi.org/10.1093/nar/gki458
Sucheta S, Harman D, Giridhar S (2001) Interaction of phenolic compounds with pectinases from Sclerotinia sclertiorum. Indian Phytopathol 54(2):167–170
Thomma BPHJ (2003) Alternaria spp.: from general saprophyte to specific parasite. Mol Plant Pathol 4(4):225–236. https://doi.org/10.1046/j.1364-3703.2003.00173.x
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882. https://doi.org/10.1093/nar/25.24.4876
Tillman RW, Siegel MR, Long JW (1973) Mechanism of action and fate of the fungicide chlorothalonil (2,4,5,6-tetrachloroisophthalonitrile) in biological systems. I. Reactions with cells and subcellular components of Saccharomyces pastorianus. Pesticide Biochem Physiol 3:160–167. https://doi.org/10.1016/0048-3575(73)90100-4
Tsuji G, Kenmochi Y, Takano Y, Sweigard J, Farrall L, Furusawa I, Horino O, Kubo Y (2000) Novel fungal transcriptional activators, Cmr1p of Colletotrichum lagenarium and pig1p of Magnaporthe grisea, contain Cys2His2 zinc finger and Zn(II)2Cys6 binuclear cluster DNA-binding motifs and regulate transcription of melanin biosynthesis genes in a developmentally specific manner. Mol Microbiol 38:940–954. https://doi.org/10.1046/j.1365-2958.2000.02181.x
van der Does HC, Rep M (2017) Adaptation to the host environment by plant-pathogenic fungi. Annu Rev Phytopathol 55:427–450. https://doi.org/10.1146/annurev-phyto-080516-035551
Wang MS, Hou X, Wang HZ (2021a) Genomic sequence resource of Alternaria alternata strain B3 causing leaf blight on Ophiopogon japonicus. Plant Dis 105(3):684–687. https://doi.org/10.1094/PDIS-07-20-1454-A
Wang XL, Lu DX, Tian CM (2021b) Analysis of melanin biosynthesis in the plant pathogenic fungus Colletotrichum gloeosporioides. Fungal Biol 125(9):679–692. https://doi.org/10.1016/j.funbio.2021.04.004
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46(W1):W296–W303. https://doi.org/10.1093/nar/gky427
Westman AL, Kresovich S, Dickson MH (1999) Regional variation in Brassica nigra and other weedy crucifers for disease reaction to Alternaria brassicicola and Xanthomonas campestris pv. campestris. Euphytica 106:253–259. https://doi.org/10.1023/A:1003544025146
Woudenberg JH, Groenewald JZ, Binder M, Crous PW (2013) Alternaria redefined. Stud Mycol 75(1):171–212. https://doi.org/10.3114/sim0015
Xu H, Zhang Q, Cui W, Zhang X, Liu W, Zhang L, Islam MN, Baek KH, Wang Y (2016) AbSte7, a MAPKK gene of Alternaria brassicicola, is involved in conidiation, salt/oxidative stress, and pathogenicity. J Microbiol Biotechnol 26(7):1311–1319. https://doi.org/10.4014/jmb.1603.03048
Yago JI, Lin CH, Chung KR (2021) The SLT2 mitogen-activated protein kinase-mediated signalling pathway governs conidiation, morphogenesis, fungal virulence and production of toxin and melanin in the tangerine pathotype of Alternaria alternata. Mol Plant Pathol 12(7):653–665. https://doi.org/10.1111/j.1364-3703.2010.00701.x
Yang ZH (1997) PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci 13:555–556. https://doi.org/10.1093/bioinformatics/13.5.555
Yang ZH, Bielawski JP (2000) Statistical methods for detecting molecular adaptation. Trends Ecol Evol 15:496–503. https://doi.org/10.1016/s0169-5347(00)01994-7
Yang Z, Nielsen R, Goldman N, Pedersen AM (2000) Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics 155:431–449. https://doi.org/10.1093/genetics/155.1.431
Yang YY, Li MR, Bao LL, He YM (2012) Study of extracting condition of fungal melanin. J Anhui Agri Sci (Chinese) 40(29):14161–14162
Yang SL, Lin CH, Chung KR (2009) Coordinate control of oxidative stress tolerance, vegetative growth, and fungal pathogenicity via the AP1 pathway in the rough lemon pathotype of Alternaria alternata. Physiol Mol Plant P 74(2):100–110. https://doi.org/10.1016/j.pmpp.2009.09.007
Yu JH, Hamari Z, Han KH, Seo JA, Reyes-Domínguez YR, Scazzocchio C (2004) Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet Biol 41:973–981. https://doi.org/10.1016/j.fgb.2004.08.001
Yu X, Huo L, Liu H, Chen LF, Wang Y, Zhu XD (2015) Melanin is required for the formation of the multi-cellular conidia in the endophytic fungus Pestalotiopsis microspore. Microbiol Res 179:1–11. https://doi.org/10.1016/j.micres.2015.06.004
Zhang A, Lu P, Dahl-Roshak AM, Paress PS, Kennedy S, Tkacz JS, An Z (2003) Efficient disruption of a polyketide synthase gene (pks1) required for melanin synthesis through Agrobacterium-mediated transformation of Glarea lozoyensis. Mol Genet Genomics 268(5):645–655. https://doi.org/10.1007/s00438-002-0780-4
Zhang CH, He YF, Zhu PK, Chen L, Wang YW, Ni B, Xu L (2015) Loss of bcbrn1 and bcpks13 in Botrytis cinerea not only blocks melanization but also increases vegetative growth and virulence. Mol Plant Microbe Interact 28(10):1091–1101. https://doi.org/10.1094/MPMI-04-15-0085-R
Zhang X, Wang Z, Jiang C, Xu JR (2021) Regulation of biotic interactions and responses to abiotic stresses by MAP kinase pathways in plant pathogenic fungi. Stress Biol 1(1):5. https://doi.org/10.1007/s44154-021-00004-3
Zhang ZX, Jia H, Liu N, Li HX, Meng QJ, Wu N, Cao ZY, Dong JG (2022) The zinc finger protein StMR1 affects the pathogenicity and melanin synthesis of Setosphaeria turcica and directly regulates the expression of DHN melanin synthesis pathway genes. Mol Microbiol 117(2):261–273. https://doi.org/10.1111/mmi.14786
Zhou YJ, Yang L, Wu MD, Chen WD, Li GQ, Zhang J (2017) A single-nucleotide deletion in the transcription factor gene bcsmr1 causes sclerotial-melanogenesis deficiency in Botrytis cinerea. Front Microbiol 8:2492. https://doi.org/10.3389/fmicb.2017.02492
Zhou YJ, Song JJ, Wang YC, Yang L, Wu MD, Li GQ, Zhang J (2022) Biological characterization of the melanin biosynthesis gene Bcscd1 in the plant pathogenic fungus Botrytis cinerea. Fungal Genet Biol 160:103693. https://doi.org/10.1016/j.fgb.2022.103693
Funding
This study was funded by the Anhui Provincial Natural Science Foundation (Grant number 1808085MC67 to Z.L.), the National Natural Science Foundation of China (Grant number 31972227 to Z.L.), the High-level Talent Subsidy Project Supported by Education Department of Anhui Province (Grant to Z.L.), and the Startup Fund for High-level Talents Sponsored by Anhui Agricultural University (Grant number 0601510 to Z.L.).
Author information
Authors and Affiliations
Contributions
Z.L. and C.Q. conceived and designed the research. C.Q. performed the experiments, analyzed the data, and wrote the manuscript. C.Q. and Z.L. revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qiu, C., Liu, Z. Positive selection and functional diversification of transcription factor Cmr1 homologs in Alternaria. Appl Microbiol Biotechnol 108, 133 (2024). https://doi.org/10.1007/s00253-023-12893-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-023-12893-7