Abstract
Bacteria have evolved an array of adaptive mechanisms enabling them to survive and grow in the presence of different environmental stresses. These mechanisms include either modifications of the membrane or changes in the overall energy status, cell morphology, and cell surface properties. Long-term adaptations are dependent on transcriptional regulation, the induction of anabolic pathways, and cell growth. However, to survive sudden environmental changes, bacterial short-term responses are essential to keep the cells alive after the occurrence of an environmental stress factor such as heat shock or the presence of toxic organic solvents. Thus far, two main short-term responses are known. On the one hand, a fast isomerization of cis into trans unsaturated fatty leads to a quick rigidification of the cell membrane, a mechanism known in some genera of Gram-negative bacteria. On the other hand, a fast, effective, and ubiquitously present countermeasure is the release of outer membrane vesicles (OMVs) from the cell surface leading to a rapid increase in cell surface hydrophobicity and finally to the formation of cell aggregates and biofilms. These immediate response mechanisms just allow the bacteria to stay physiologically active and to employ long-term responses to assure viability upon changing environmental conditions. Here, we provide insight into the two aforementioned rapid adaptive mechanisms affecting ultimately the cell envelope of Gram-negative bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The envelope of Gram-negative bacteria is the outermost barrier of the cell, and it consists of an outer membrane with lipopolysaccharides (LPS) facing the extracellular space and an inner membrane enclosing the cytoplasm. The space between these membranes, known as periplasm, contains a peptidoglycan layer. While the outer membrane is not permeable throughout most of its surface, non-specific porins allow the passive diffusion of molecules (Hancock et al. 1979; Nakae 1976; van den Berg 2012). The inner membrane represents the main diffusion barrier of the Gram-negative cell, and it is very susceptible to stressors like hydrocarbons or elevated temperatures. Both stressors increase directly the membrane fluidity, either due to the accumulation of hydrophobic substances in the membrane or due to an increased mobility of phospholipids (Beney and Gervais 2001; Hazel and Williams 1990; Heipieper and de Bont 1994). This can lead to the disruption of essential functions of the membrane as enzymatic matrix and diffusion barrier resulting in the collapse of electrochemical gradients (Isken and de Bont 1998). To maintain a certain degree of membrane fluidity, the membrane composition can be altered, a process known as homeoviscous adaptation (Sinensky 1974). To counteract increasing membrane fluidity, the synthesis of saturated phospholipid fatty acids is a common mechanism in Gram-negative bacteria (Ingram 1977; Kabelitz et al. 2003; Suutari and Laakso 1994). Phospholipids containing saturated fatty acids display a much higher transition temperature than those with unsaturated fatty acids, thereby decreasing membrane fluidity. For example the melting point of phospholipids with C16:0 is about 63 °C higher than that of phospholipids with 16:1 cis-unsaturated fatty acids (Roach et al. 2004; Zhang and Rock 2008). However, in a situation in which the bacterial cell is facing severe and fast environmental changes (e.g. high concentrations of toxic substances) resulting in elevated mobility of membrane components, the comparatively slow-acting de novo synthesis of saturated fatty acids cannot keep up. Moreover, the de novo synthesis of saturated fatty acids is an energy-consuming process which cannot be employed in conditions where cell viability is impaired (Heipieper et al. 2007). Consequently, the survival of the cell depends on adaption mechanisms acting immediately and which are independent from transcriptional regulation and growth. Notably, these mechanisms ensure the survival until growth-dependent mechanisms complete the adaptive response (Cronan 2002; Hartig et al. 2005; Heipieper et al. 2007; Zhang and Rock 2008). As the cell envelope of Gram-negative bacteria is affected first by emerging environmental stress, both fast adaptive responses described in the following sections involve changes of the interface between the cell and its surrounding. It is important to notice that the inner as well as the outer membrane can be altered by mechanisms taking effect fast but differ fundamentally in their mode of action. Which of the two membranes could be modified by which mechanisms and how will be explained and discussed below.
Cis-trans-isomerization of membrane fatty acids
The cytoplasmic membrane of most Gram-negative bacteria consists of saturated and monounsaturated fatty acids. For a long time, it was believed that unsaturated membrane fatty acids always exhibit the cis configuration, until in the mid-1980s evidence for the existence of trans unsaturated fatty acids in Vibrio and Pseudomonas was presented (Guckert et al. 1986; Guckert et al. 1987). Ultimately, it was discovered that the conversion of cis unsaturated fatty acids into their corresponding trans configuration represents a strategy to withstand elevated temperatures in Vibrio sp. strain ABE-1 and toxic hydrocarbons in Pseudomonas putida P8 (Heipieper et al. 1992; Okuyama et al. 1991). In both organisms, the amount of trans fatty acids was increased by simultaneous decrease of their cis form. In P. putida P8, it could be shown that the amount of trans fatty acids correlates positively with the concentration of the stressor phenol added to the cultures and even non-dividing cells showed the conversion of cis to trans (Heipieper et al. 1992). Further, the cis-trans conversion is not affected by inhibition of the fatty acid biosynthesis with cerulenin and is independent from the de novo synthesis of proteins (Heipieper et al. 1992; Kiran et al. 2005). Therefore, the cis-trans conversion is a powerful tool during growth-inhibiting conditions where the general fatty acid composition cannot be changed (Morita et al. 1993). The applicability as a fast adaptive response is conferred by the fact that it works constitutively and that highest amounts of the trans fatty acids are accumulated already 30 min after the initiated stress. Moreover, neither ATP nor cofactors are required to perform the cis-trans conversion (von Wallbrunn et al. 2003). Furthermore, cis-trans conversion is not affected by chloramphenicol, which blocks protein translation. Hence, the cis-trans isomerization is independent from the de novo protein biosynthesis (Heipieper et al. 1992; Kiran et al. 2005).
The conversion from cis to trans fatty acids is carried out by the periplasmic protein Cti, the cis-trans-isomerase. Predominant substrates for the enzyme are the fatty acid residues palmitoleic acid (C16:1Δ9cis) and cis-vaccenic acid (C18:1Δ11cis) of phospholipids (Heipieper et al. 1992; Heipieper et al. 2003). The position of the double bond remains the same after the reaction. This was shown when growth media of P. putida was supplemented with oleic acid (C18:1Δ9cis). This fatty acid is not naturally produced by P. putida but incorporated into the phospholipids of the membrane. After exposure to solvent stress with 4-chlorophenol, the direct conversion to the corresponding trans isomer elaidic acid (C18:1Δ9trans) took place and oleic acid was depleted (Diefenbach and Keweloh 1994). Further proof for that the double bond remains at the same position during the isomerization was provided by the fact that the sum of the amount of both isomers kept constant regardless of the toxin concentration (Heipieper et al. 1992). But how exactly contributes an increased trans/cis ratio to a more rigid membrane? Under non-stressed conditions, unsaturated fatty acids exhibit the cis configuration, and the double bond present displays an unmovable bend of 30° (Macdonald et al. 1985; Roach et al. 2004). This kink leads to steric hindrance within the fatty acid residues enhancing membrane fluidity. In contrast, in trans unsaturated fatty acids, the kink adds up to only 6° (Macdonald et al. 1985). Thus, compared to the cis isomers, trans fatty acids can align more closely to each other and in this regard resemble saturated fatty acid residues (Heipieper et al. 2003; Kulig et al. 2016; Pierce et al. 2014). Consequently, this results in a more rigid membrane (Chen et al. 1995; Diefenbach et al. 1992). This was also demonstrated by comparing the behavior of phospholipids containing trans fatty acids with those containing the corresponding cis fatty acids in model membranes and by molecular dynamics (Roach et al. 2004).
Cis-trans-isomerization can be induced by the accumulation of different hydrocarbons and phenolic compounds in the cytoplasmic membrane. Their ability to accumulate in the membrane depends on their hydrophobicity given as log Pow value. This value is the partitioning coefficient of a compound between octanol and water (Laane et al. 1987). Organic hydrocarbons with a Pow value between 1 and 4 preferentially partition inside the membrane and thus are highly toxic for microorganisms (Heipieper and de Bont 1994; Sikkema et al. 1995; Weber and de Bont 1996). Notably, the degree of cis to trans conversion correlates with the toxicity/hydrophobicity and the concentration of toxic organic hydrocarbons but does not depend on a distinctive chemical structure of the stressor (Heipieper et al. 1995; Neumann et al. 2005). Besides various toxic organic compounds, osmotic stress, the presence of heavy metals, and antibiotics acting on the membrane result in an increase of the trans/cis ratio (Hachicho et al. 2014; Heipieper et al. 1996; Isken et al. 1997; Kotchaplai et al. 2017; Piotrowska et al. 2016). This reflects the importance of the system as general stress response mechanism in Gram-negative bacteria (Ramos et al. 2001; Segura et al. 1999; Zhang and Rock 2008). In contrast to that, Cti cannot be employed to adjust the membrane fluidity if temperatures drop because the conversion from trans to cis is not catalyzed but requires the de novo synthesis of unsaturated fatty acids, and the amount of trans fatty acids is low in Gram-negative bacteria. It has to be mentioned that the effectiveness of cis-trans-isomerization is not as high as the replacement of unsaturated fatty acids by saturated ones (Macdonald et al. 1985; Roach et al. 2004). But, especially if a fast reaction to environmental stress is needed, the conversion of cis fatty acids contributes substantially to the survival of the cell and allows subsequent long-term adaptations to take place. This has been demonstrated for heat shock conditions and the exposure of cells to toxic hydrocarbons (Heipieper et al. 2003; Kiran et al. 2005; Weber and de Bont 1996).
So far, cis-trans-isomerization was shown to be employed as adaptive response to stress in strains of all known Pseudomonas sp. (Heipieper et al. 1992; Molina-Santiago et al. 2017), Vibrio sp. (Okuyama et al. 1991), Methylococcus capsulatus (Löffler et al. 2010), Alcanivorax borkumensis (Naether et al. 2013), and Colwellia psychrerythraea (Hashimoto et al. 2015). Table 1 summarizes the results of a recent BLASTP analysis using the predicted amino acid sequence from the cti gene of P. aeruginosa PAO1 as reference and reveals that the system might also be present in other microorganisms. Although physiological evidence is not yet provided, a promising candidate for bearing cis-trans-isomerization activity is Nitrosomonas since the genera contains trans fatty acid residues in its phospholipids (Keweloh and Heipieper 1996). By performing alignments of the peptide sequence, it was found that almost all Cti proteins bear an N-terminal signal sequence (except the one from Vibrio cholera) suggesting a periplasmic location of the enzyme; this was confirmed for P. oleovorans and P. putida DOT-T1E (Junker and Ramos 1999; Pedrotta and Witholt 1999). Phylogenetic analysis revealed that Cti proteins from the genera Pseudomonas and Vibrio diversified into three branches from a common ancestor. The largest group is composed out of proteins from P. putida, P. aeruginosa, Pseudomonas sp., and V. cholerae while the latter clearly emanates from the others (von Wallbrunn et al. 2003). Although the effective and simple cis-trans-isomerization is not a common feature present in Gram-negative bacteria, it presumably contributes to the high adaptability of particularly Pseudomonas and Vibrio species conquering diverse ecosystems and showing a high durability in environmental adverse situations. This idea is supported by the increased tolerance towards octanoic acid of a genetically modified Escherichia coli strain containing the Cti from P. aeruginosa (Tan et al. 2016). It is noteworthy that the expression of cti improved the robustness of the E. coli strain and concomitantly facilitates biotechnological downstream processing. For biochemical characterization, the Cti protein from P. putida P8 was recombinantly expressed in E. coli (Holtwick et al. 1997) or purified from periplasmic fractions of P. oleovorans and Pseudomonas sp. E-3 (Okuyama et al. 1998; Pedrotta and Witholt 1999). Cti is a neutral 87 kDa protein, monocistronically transcribed and continuously expressed (Kiran et al. 2005). After translocating the protein to the periplasm, the N-terminal hydrophobic signal sequence is cleaved off (Holtwick et al. 1997; Junker and Ramos 1999; Pedrotta and Witholt 1999). Cti is a cytochrome c-type protein as it is bearing a heme-binding motif (Holtwick et al. 1999). For P. putida, it was proposed that iron (probably Fe3+) is essential for the reaction catalyzed (Okuyama et al. 1998). This is in agreement with observations of strongly diminished Cti activity after site-directed mutagenesis of the heme-binding site as well as shutting down the assembly machinery of heme groups to cytochrome c-type proteins in P. putida P8 (Holtwick et al. 1999). Via the putative Fe3+ coordinated by the heme-binding site, an enzyme substrate complex is formed and an electron is temporarily removed from the cis-double bond through electrophilic attack changing the sp2 hybridization to sp3, thus enabling the free rotation of the C–C bond. After establishing the trans configuration, the electron is transferred back reforming the double bond (Okuyama et al. 1998; von Wallbrunn et al. 2003). Other cofactors like NAD(P)H, glutathione, or ATP are not necessary to carry out the reaction (Diefenbach et al. 1992; Heipieper and de Bont 1994; Heipieper et al. 1992).
Characteristically for immediate response mechanisms, no transcriptional regulation exists for Cti (Kiran et al. 2005). Instead, the activity of the constitutively expressed enzyme has to be regulated differently. Cells deprived in energy sources and also non-dividing cells show cis-trans-isomerization; hence, a complex model for regulation involving enzymatic pathways can presumably be ruled out (Heipieper et al. 1992). The regulation is probably simply done by enabling the access of the enzyme to its substrate, which can be controlled via membrane fluidity (Fig. 1). Under non-stressed conditions, the membrane is relatively rigid and cis fatty acid residues of phospholipids are not accessible for the hydrophilic Cti since they are embedded in a certain depth of the membrane. The presence of hydrocarbons partitioning in the membrane or high temperature increases the membrane’s fluidity substantially (Hartig et al. 2005; Heipieper et al. 2001). In this process, gaps in a more disordered membrane open up allowing Cti to bind to and react with its substrate. As more cis fatty acid residues are converted to trans, acyl chains are packed tighter, thereby pushing Cti out of the membrane (Chen et al. 1995; Loffhagen et al. 2007; Roach et al. 2004; Seelig and Waespe-Sarcevic 1978). In other words, a certain fluidity of the membrane is necessary for Cti to reach its substrate inside the membrane and reduction of membrane fluidity due to cis-trans-isomerization counteracts the intrusion of the isomerase (Heipieper et al. 2003; Heipieper et al. 1996). This model explains the observed correlation between the extent of cis-trans-isomerization and the toxicity caused by different concentrations of specific environmental stressors (Heipieper et al. 1995; Heipieper et al. 1996). In addition, the model is in accordance with the fast onset of this regulatory mechanism, and as soon as the membrane fluidity surpasses or falls below a certain threshold, Cti becomes active or inactive respectively (Heipieper et al. 2001).
The application of the trans/cis ratio and the formation of trans unsaturated fatty acids as stress biomarker in environmental samples or during in situ bioremediation processes was discussed, and it was proposed that a trans/cis ratio higher than 0.1 could indicate the occurrence of environmental stress (Guckert et al. 1986; Guckert et al. 1987; Heipieper et al. 1995; Pinkart et al. 1996). However, it was proven that an increased amount of trans unsaturated fatty acids may not be a suitable benchmark to evaluate environmental stress scenarios, especially for long-term contaminated sites (Fischer et al. 2010). Due to its nature as urgent stress response, an increased trans/cis ratio can be measured directly after a contamination event, but with the onset of long-term adaptations, the trans/cis ratio decreases to levels of an unstressed reference (Fischer et al. 2010). Moreover, it is not possible to assign whether a changing phospholipid fatty acid pattern is derived from alterations on the membrane or the species level (Frostegard et al. 2011).
Formation of OMVs
Besides adapting the inner membrane in response to environmental stress, Gram-negative bacteria have evolved defense mechanisms altering the outer membrane. The outer membrane consists of a distal lipopolysaccharide (LPS) layer and a corresponding proximal phospholipid monolayer, which includes linkers to the peptidoglycan layer in the periplasm. Characteristic for the LPS layer are three different components: lipid A, an amphipathic glycolipid; the core oligosaccharide; and the most distal, the O-specific polysaccharide (also known as O-antigen) region. Compared to the inner membrane, adaptive mechanisms affecting the outer membrane are far less understood (de Carvalho et al. 2009; Heipieper et al. 1991; Neumann et al. 2006). However, the formation of outer membrane vesicles (OMVs), which are spherical particles with a diameter between 20 and 500 nm released from the outer membrane into the extracellular space, has been studied in detail (Schwechheimer and Kuehn 2015, Beveridge 1999; Kuehn and Kesty 2005; Mashburn-Warren et al. 2008b). The release of OMVs is a common phenomenon among Gram-negative bacteria (Kulp and Kuehn 2010; Schwechheimer et al. 2013; Zhou et al. 1998), and it is attributed to the pathogenicity of strains like P. aeruginosa causing cystic fibrosis (Kadurugamuwa and Beveridge 1997). Moreover, Gram-negative bacteria forming OMVs are present in any environment, and sessile as well as planktonic cells have been found to release OMVs (Beveridge 1999; Beveridge et al. 1997; Biller et al. 2014; Brandtzaeg et al. 1992; Hellman et al. 2000; Hickey et al. 2015). The biogenesis of OMVs and their numerous functions besides stress adaptation have been recently reviewed in great detail (Kulp and Kuehn 2010; Schertzer and Whiteley 2013; Schwechheimer and Kuehn 2015). The function of OMV release as a stress response was shown in several reports. For example, when P. aeruginosa was treated with the antibiotic ciprofloxacin or hydrogen peroxide, the bacteria responded with increased vesicle production (Macdonald and Kuehn 2013; Maredia et al. 2012). In addition, the production of OMVs in P. putida as response to the exposure to solvents, high temperature, and EDTA was shown before (Baumgarten et al. 2012b; Baumgarten et al. 2012a). The reason why the formation of OMV is beneficial during stress conditions will be approached and explained in the following section.
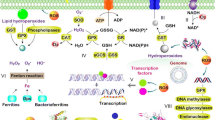
Certain stress responses in Gram-negative bacteria can be found in many genera, potentially being present ubiquitously (Heipieper et al. 2007; Ramos et al. 2001; Segura et al. 1999), and the modification of bacterial surface properties like surface charge and hydrophobicity is one of those (Wick et al. 2003; Wick et al. 2002). The latter can be measured as the contact angle (θw) between a bacterial lawn and a droplet of water (van Loosdrecht et al. 1987). In several bacteria, an increase in the surface hydrophobicity was observed when cells were incubated with solvents or exposed to osmotic stress (Baumgarten et al. 2012a; Hachicho et al. 2017; Löffler et al. 2010; Naether et al. 2013; Neumann et al. 2006; Wick et al. 2002). This enhanced hydrophobicity was observed to be a rapid response (within 10 min) and was also proven to be of physiological origin since dead cells did not show such a reaction. Moreover, after withdrawal of the solvent, the hydrophobicity recovered slowly but steadily to its previous status most probably due to the de novo synthesis of LPS components (Neumann et al. 2006). In P. aeruginosa, the O-specific region of the LPS layer contains two major components (Makin and Beveridge 1996). The A-band is a rather hydrophobic and low-molecular-weight LPS, consisting of a homopolymer of d-rhamnose and small amounts of 2-keto-3-deoxyoctonic acid. The second component, the B-band, is a rather hydrophilic and high-molecular-weight LPS, consisting of a heteropolymer of mainly uronic acid derivatives and N-acetylfucosamine (Sabra et al. 2003). In P. aeruginosa, a depletion of B-band components in the LPS layer was observed after exposure to high temperature, membrane-active antibiotics, or at low oxygen saturation (Makin and Beveridge 1996) and a similar response was reported for P. putida after exposure to o-xylene (Pinkart et al. 1996). The assumption that the depletion of the B-band is derived from its export within OMVs is supported by several observations. In P. aeruginosa, formation of OMVs was reported after treatment with hydrogen peroxide and vesiculation was dependent on the ability of the bacterium to form B-band LPS (Macdonald and Kuehn 2013). Moreover, an enrichment of B-band LPS in the constitutively produced OMVs of P. aeruginosa was documented earlier (Li et al. 1996). Consistent with that is the report of hypersensitivity towards oxidative stress caused by the deficiency to synthesize B-band LPS in Gram-negative Enterobacterium (Berry et al. 2009). In addition, a number of reports state that OMVs formed in cultures of P. aeruginosa were enriched with certain LPS components after stress exposure (Berry et al. 2009; Deatherage et al. 2009; Kadurugamuwa and Beveridge 1995; Sabra et al. 2003; Schooling and Beveridge 2006). Taken together, the hydrophilic B-band LPS is preferably exported via OMVs whereas the hydrophobic A-band LPS remains in the bacterial outer membrane. This means that the amount of OMVs produced determines the increase in cell surface hydrophobicity. A conceivable opinion that solvents are repelled with a more hydrophilic surface (de Bont 1998) is obviously invalid, but what is the advantage of a hydrophobic surface? Recently, cultures of P. putida stressed with solvents, NaCl, EDTA, or heat were proven to have an enhanced ability to form biofilms after increasing their hydrophobicity (Baumgarten et al. 2012b). Moreover, the OMVs detected in the culture supernatants after application of stress showed proteomic and lipidomic composition typical for the outer membrane. That OMVs could be involved in biofilm formation was suggested before (Beveridge et al. 1997). Biofilms or microcolonies are microbial agglomerations embedded in extracellular polymeric substances (EPSs), attached to surfaces and displaying a different phenotype than planktonic cells (Costerton et al. 1978; Donlan and Costerton 2002). The major steps in biofilm formation are adhesion of planktonic cells to the surface; the initiation and maturation of the agglomeration; and finally, the dispersion of cells from the biofilm (Stoodley et al. 2002). Bacteria growing in biofilms are known to have considerable advantages over the planktonic lifestyle. Bacterial cells are protected within the biofilm; hence, the capability to withstand and tolerate harsh environmental conditions as treatment with antimicrobial compounds, heat, or solvents is increased (Beveridge et al. 1997; Ceri et al. 1999; de Carvalho et al. 2009; Kim et al. 2012; Weitere et al. 2005). The biofilm consists out of cells, EPS, proteins, DNA, and other macromolecules (Sutherland 2001) but also OMVs contribute to the biofilm matrix (Beveridge et al. 1997; Schertzer and Whiteley 2012; Schooling and Beveridge 2006). In addition to the stabilization of the biofilm, its formation is also enhanced a lot due to the fact that cells forming vesicles turn more hydrophobic as discussed above. Subsequently, this favors an attachment of cells to each other and to a hydrophobic surface. To sum up, Fig. 2 shows a general stress response cascade in Gram-negative bacteria that is most likely initiated by vesiculation conferring a higher surface hydrophobicity to the cells that subsequently results in an enhanced ability to form biofilms or microcolonies (Baumgarten et al. 2012b).
Scheme of Gram-negative cell wall with membrane micro domain associated LPS types. The formation of outer membrane vesicles from a certain membrane micro domain is shown. 1 Environmental stress (e.g., temperature, solvents, EDTA, osmotic stress) on bacterial outer membrane. 2 Subsequent bulging out of the outer membrane and vesicle detachment resulting in a depletion of hydrophilic LPS type (blue). 3 Concomitant enrichment of hydrophobic LPS type (yellow) in the outer membrane leads to enhanced ability to form biofilms
Various theories how OMVs are formed are currently discussed in literature (Schwechheimer and Kuehn 2015). In this regard, one of the best investigated strains is P. aeruginosa because of its importance as pathogen causing cystic fibrosis. Here, the conversion of 2-heptyl-4-quinolone (HHQ) into 2-heptyl-3-hydroxy-4-quinolone, the so-called Pseudomonas quinolone signal (PQS), by the cytoplasmic monooxygenase PqsH and the subsequent export of PQS stimulates the formation of OMVs (Diggle et al. 2006; Florez et al. 2017; Schertzer et al. 2010). PQS has a heteroaromatic structure with a C7 aliphatic residue. Due to its amphiphilic character, the molecule interacts with both the 4′-phosphate and acyl chains of lipid A (Mashburn-Warren et al. 2008a) and thus can accumulate in the LPS layer promoting vesicle blebbing (Schertzer et al. 2009). In addition, in P. aeruginosa, a sequestration of the PQS molecule in the inner membrane, which could lead to rapid OMV production once further forwarded to the outer membrane, was discussed (Florez et al. 2017). Vesiculation induced specifically by PQS seems not to be a general feature among Gram-negative bacteria since already among the same genus, the strain Pseudomonas putida KT2440 does have neither any homologous genes for the synthesis of PQS nor any other known quorum sensing molecule but seems to regulate population density through a so far unknown signal molecule (Espinosa-Urgel and Ramos 2004; Fernandez-Pinar et al. 2008). Trace amounts of HHQ were detected in P. putida culture supernatants but with concentrations at least one order of magnitude lower than in P. aeruginosa (Dubern and Diggle 2008). Nevertheless, supported by the fast formation of OMVs in P. putida, P. aeruginosa, and E. coli (Baumgarten et al. 2012a; Makin and Beveridge 1996; Manning and Kuehn 2011; Neumann et al. 2006), the existence of several different signal molecules or other mechanisms seems plausible to secure the ability to act immediately upon emerging environmental stress. Hereby, amphipathic molecules able to penetrate the outer membrane are conceivable candidates. Moreover, the existence of hotspots for vesiculation would explain how such a process is controlled without inducing membrane blebbing all over the bacterial surface with concomitant risk of cell lysis. Consistent with that is a more recent understanding of the membrane displaying a rather heterogeneous than homogeneous pattern. So-called membrane micro domains (also known as lipid rafts) may be formed by an enrichment of certain proteins or glycolipids (Bramkamp and Lopez 2015; Engelman 2005). Membrane micro domains where OMV formation preferably originates could contain an enrichment of B-band LPS. Diminished crosslinks between the outer membrane and the peptidoglycan (Kulp and Kuehn 2010; McBroom et al. 2006; Schwechheimer and Kuehn 2013; Wessel et al. 2013) would further promote the vesiculation process.
Since both adaptive mechanisms act on the Gram-negative cell envelope at first, it may not always be clear which effect can be traced back to which mechanism. However, since the outer membrane is of asymmetric architecture, the number of phospholipids is much smaller than in the inner membrane. Hence, the targets for Cti are less abundant and in turn resulting the cytoplasmic membrane to be the main domain affected by cis-trans-isomerization. Nonetheless, the fatty acid composition of OMV preparations of solvent-stressed cells revealed an elevated trans/cis ratio indicating that the Cti is also acting in part on the outer membrane (Baumgarten et al. 2012b). To elucidate this further would be an interesting research topic and also would tell more about Cti’s scope of action.
In summary, the formation of OMVs is a ubiquitous mechanism in Gram-negative bacteria. Presumably, a basal production of vesicles is always present and vesiculation can be increased in certain scenarios (Schwechheimer and Kuehn 2015). To elucidate the probably diverse mechanisms of vesiculation, its regulation remains a challenging research field. As proposed for cis-trans-isomerization (Heipieper et al. 2003; Heipieper et al. 1996) also for OMV formation, a rather simple trigger than a complex regulation is conceivable to assure that the cell can immediately act independent of growth and energy supply. In bacteria bearing both adaptational mechanisms, most probably, they take place at the same time in a stress situation due to their passive activation. This phenomenon, a concomitant increase in surface hydrophobicity as well as for the trans/cis ratio, was already observed for bacteria in stress situations (Löffler et al. 2010; Naether et al. 2013). However, in contrast to cis-trans-isomerization, it was demonstrated that neither non-growing cells nor dead cells could increase their hydrophobicity fast and efficient upon stress exposure or showed an enhanced ability to form biofilms (Baumgarten et al. 2012b; Neumann et al. 2006). Both urgent response systems, the cis-trans-isomerization as well as the formation of OMVs, secure a fast alteration and adaptation in the Gram-negative bacteria cell envelope to prevent a deadly impact of emerging adverse environmental conditions. Concomitantly, both responses can provide sufficient time for further more complex but also slower-acting adaptive cascades eventually completing the adaptation process (Cronan 2002; Hartig et al. 2005; Zhang and Rock 2008).
References
Baumgarten T, Sperling S, Seifert J, von Bergen M, Steiniger F, Wick LY, Heipieper HJ (2012b) Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Appl Environ Microbiol 78(17):6217–6224. https://doi.org/10.1128/AEM.01525-12
Baumgarten T, Vazquez J, Bastisch C, Veron W, Feuilloley MG, Nietzsche S, Wick LY, Heipieper HJ (2012a) Alkanols and chlorophenols cause different physiological adaptive responses on the level of cell surface properties and membrane vesicle formation in Pseudomonas putida DOT-T1E. Appl Microbiol Biotechnol 93(2):837–845. https://doi.org/10.1007/s00253-011-3442-9
Beney L, Gervais P (2001) Influence of the fluidity of the membrane on the response of microorganisms to environmental stresses. Appl Microbiol Biotechnol 57(1–2):34–42
Berry MC, McGhee GC, Zhao Y, Sundin GW (2009) Effect of a waaL mutation on lipopolysaccharide composition, oxidative stress survival, and virulence in Erwinia amylovora. FEMS Microbiol Lett 291(1):80–87. https://doi.org/10.1111/j.1574-6968.2008.01438.x
Beveridge TJ (1999) Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol 181(16):4725–4733
Beveridge TJ, Makin SA, Kadurugamuwa JL, Li ZS (1997) Interactions between biofilms and the environment. FEMS Microbiol Rev 20(3–4):291–303. https://doi.org/10.1111/j.1574-6976.1997.tb00315.x
Biller SJ, Schubotz F, Roggensack SE, Thompson AW, Summons RE, Chisholm SW (2014) Bacterial vesicles in marine ecosystems. Science 343(6167):183–186. https://doi.org/10.1126/science.1243457
Bramkamp M, Lopez D (2015) Exploring the existence of lipid rafts in bacteria. Microbiol Mol Biol Rev 79(1):81–100. https://doi.org/10.1128/MMBR.00036-14
Brandtzaeg P, Bryn K, Kierulf P, Ovstebo R, Namork E, Aase B, Jantzen E (1992) Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J Clin Invest 89(3):816–823. https://doi.org/10.1172/JCI115660
Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A (1999) The calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 37(6):1771–1776
Chen Q, Janssen DB, Witholt B (1995) Growth on octane alters the membrane lipid fatty-acids of Pseudomonas oleovorans due to the induction of Alkb and synthesis of octanol. J Bacteriol 177(23):6894–6901
Costerton JW, Geesey GG, Cheng KJ (1978) How bacteria stick. Sci Am 238(1):86–95
Cronan JE (2002) Phospholipid modifications in bacteria. Curr Opin Microbiol 5(2):202–205. https://doi.org/10.1016/S1369-5274(02)00297-7
de Bont JAM (1998) Solvent-tolerant bacteria in biocatalysis. Trends Biotechnol 16(12):493–499. https://doi.org/10.1016/S0167-7799(98)01234-7
de Carvalho CC, Wick LY, Heipieper HJ (2009) Cell wall adaptations of planktonic and biofilm Rhodococcus erythropolis cells to growth on C5 to C16 n-alkane hydrocarbons. Appl Microbiol Biotechnol 82(2):311–320. https://doi.org/10.1007/s00253-008-1809-3
Deatherage BL, Lara JC, Bergsbaken T, Rassoulian Barrett SL, Lara S, Cookson BT (2009) Biogenesis of bacterial membrane vesicles. Mol Microbiol 72(6):1395–1407. https://doi.org/10.1111/j.1365-2958.2009.06731.x
Diefenbach R, Heipieper HJ, Keweloh H (1992) The conversion of cis into trans unsaturated fatty acids in Pseudomonas putita P8-evidence for a role in the regulation of membrane fluidity. Appl Microbiol Biotechnol 38(3):382–387
Diefenbach R, Keweloh H (1994) Synthesis of trans unsaturated fatty acids in Pseudomonas putida P8 by direct isomerization of the double bond of lipids. Arch Microbiol 162(1–2):120–125
Diggle SP, Lumjiaktase P, Dipilato F, Winzer K, Kunakorn M, Barrett DA, Chhabra SR, Camara M, Williams P (2006) Functional genetic analysis reveals a 2-Alkyl-4-quinolone signaling system in the human pathogen Burkholderia pseudomallei and related bacteria. Chem Biol 13(7):701–710. https://doi.org/10.1016/j.chembiol.2006.05.006
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15(2):167–193. https://doi.org/10.1128/Cmr.15.2.167-193.2002
Dubern JF, Diggle SP (2008) Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Mol BioSyst 4(9):882–888. https://doi.org/10.1039/b803796p
Engelman DM (2005) Membranes are more mosaic than fluid. Nature 438(7068):578–580. https://doi.org/10.1038/nature04394
Espinosa-Urgel M, Ramos JL (2004) Cell density-dependent gene contributes to efficient seed colonization by Pseudomonas putida KT2440. Appl Environ Microbiol 70(9):5190–5198. https://doi.org/10.1128/AEM.70.9.5190-5198.2004
Fernandez-Pinar R, Ramos JL, Rodriguez-Herva JJ, Espinosa-Urgel M (2008) A two-component regulatory system integrates redox state and population density sensing in Pseudomonas putida. J Bacteriol 190(23):7666–7674. https://doi.org/10.1128/JB.00868-08
Fischer J, Schauer F, Heipieper HJ (2010) The trans/cis ratio of unsaturated fatty acids is not applicable as biomarker for environmental stress in case of long-term contaminated habitats. Appl Microbiol Biotechnol 87(1):365–371. https://doi.org/10.1007/s00253-010-2544-0
Florez C, Raab JE, Cooke AC, Schertzer JW (2017) Membrane distribution of the pseudomonas quinolone signal modulates outer membrane vesicle production in Pseudomonas aeruginosa. MBio 8(4):e01034–e01017. https://doi.org/10.1128/mBio.01034-17
Frostegard A, Tunlid A, Baath E (2011) Use and misuse of PLFA measurements in soils. Soil Biol Biochem 43(8):1621–1625. https://doi.org/10.1016/j.soilbio.2010.11.021
Guckert JB, Hood MA, White DC (1986) Phospholipid ester-linked fatty acid profile changes during nutrient deprivation of Vibrio cholerae—increases in the trans cis ratio and proportions of cyclopropyl fatty acids. Appl Environ Microbiol 52(4):794–801
Guckert JB, Ringelberg DB, White DC (1987) Biosynthesis of trans fatty acids from acetate in the bacterium Pseudomonas atlantica. Can J Microbiol 33(9):748–754
Hachicho N, Birnbaum A, Heipieper HJ (2017) Osmotic stress in colony and planktonic cells of Pseudomonas putida mt-2 revealed significant differences in adaptive response mechanisms. AMB Express 7:62. https://doi.org/10.1186/s13568-017-0371-8
Hachicho N, Hoffmann P, Ahlert K, Heipieper HJ (2014) Effect of silver nanoparticles and silver ions on growth and adaptive response mechanisms of Pseudomonas putida mt-2. FEMS Microbiol Lett 355(1):71–77. https://doi.org/10.1111/1574-6968.12460
Hancock RE, Decad GM, Nikaido H (1979) Identification of the protein producing transmembrane diffusion pores in the outer membrane of Pseudomonas aeruginosa PA01. Biochim Biophys Acta 554(2):323–331
Hartig C, Loffhagen N, Harms H (2005) Formation of trans fatty acids is not involved in growth-linked membrane adaptation of Pseudomonas putida. Appl Environ Microbiol 71(4):1915–1922. https://doi.org/10.1128/AEM.71.4.1915-1922.2005
Hashimoto M, Orikasa Y, Hayashi H, Watanabe K, Yoshida K, Okuyama H (2015) Occurrence of trans monounsaturated and polyunsaturated fatty acids in Colwellia psychrerythraea strain 34H. J Basic Microb 55(7):838–845. https://doi.org/10.1002/jobm.201400815
Hazel JR, Williams EE (1990) The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res 29(3):167–227
Heipieper HJ, de Bont JAM (1994) Adaptation of Pseudomonas putida S12 to ethanol and toluene at the level of fatty acid composition of membranes. Appl Environ Microbiol 60(12):4440–4444
Heipieper HJ, de Waard P, van der Meer P, Killian JA, Isken S, de Bont JAM, Eggink G, de Wolf FA (2001) Regiospecific effect of 1-octanol on cis-trans isomerization of unsaturated fatty acids in the solvent-tolerant strain Pseudomonas putida S12. Appl Microbiol Biotechnol 57(4):541–547
Heipieper HJ, Diefenbach R, Keweloh H (1992) Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl Environ Microbiol 58(6):1847–1852
Heipieper HJ, Keweloh H, Rehm HJ (1991) Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli. Appl Environ Microbiol 57(4):1213–1217
Heipieper HJ, Loffeld B, Keweloh H, de Bont JAM (1995) The cis/trans isomerisation of unsaturated fatty acids in Pseudomonas putida S12: an indicator for environmental stress due to organic compounds. Chemosphere 30(6):1041–1051
Heipieper HJ, Meinhardt F, Segura A (2003) The cis-trans isomerase of unsaturated fatty acids in Pseudomonas and Vibrio: biochemistry, molecular biology and physiological function of a unique stress adaptive mechanism. FEMS Microbiol Lett 229(1):1–7
Heipieper HJ, Meulenbeld G, van Oirschot Q, de Bont JAM (1996) Effect of environmental factors on the trans/cis ratio of unsaturated fatty acids in Pseudomonas putida S12. Appl Environ Microbiol 62(8):2773–2777
Heipieper HJ, Neumann G, Cornelissen S, Meinhardt F (2007) Solvent-tolerant bacteria for biotransformations in two-phase fermentation systems. Appl Microbiol Biotechnol 74(5):961–973. https://doi.org/10.1007/s00253-006-0833-4
Hellman J, Loiselle PM, Zanzot EM, Allaire JE, Tehan MM, Boyle LA, Kurnick JT, Warren HS (2000) Release of gram-negative outer membrane proteins into human serum and septic rat blood and their interactions with immunoglobulin in antiserum to Escherichia coli J5. J Infect Dis 181(3):1034–1043. https://doi.org/10.1086/315302
Hickey CA, Kuhn KA, Donermeyer DL, Porter NT, Jin CS, Cameron EA, Jung H, Kaiko GE, Wegorzewska M, Malvin NP, Glowacki RWP, Hansson GC, Allen PM, Martens EC, Stappenbeck TS (2015) Colitogenic Bacteroides thetaiotaomicron antigens access host immune cells in a sulfatase-dependent manner via outer membrane vesicles. Cell Host Microbe 17(5):672–680. https://doi.org/10.1016/j.chom.2015.04.002
Holtwick R, Keweloh H, Meinhardt F (1999) cis/trans isomerase of unsaturated fatty acids of Pseudomonas putida P8: evidence for a heme protein of the cytochrome c type. Appl Environ Microbiol 65(6):2644–2649
Holtwick R, Meinhardt F, Keweloh H (1997) cis-trans isomerization of unsaturated fatty acids: cloning and sequencing of the cti gene from Pseudomonas putida P8. Appl Environ Microbiol 63(11):4292–4297
Ingram LO (1977) Changes in lipid composition of Escherichia coli resulting from growth with organic solvents and with food additives. Appl Environ Microbiol 33(5):1233–1236
Isken S, de Bont JAM (1998) Bacteria tolerant to organic solvents. Extremophiles 2(3):229–238
Isken S, Santos PMAC, de Bont JAM (1997) Effect of solvent adaptation on the antibiotic resistance in Pseudomonas putida S12. Appl Microbiol Biotechnol 48(5):642–647
Junker F, Ramos JL (1999) Involvement of the cis/trans isomerase Cti in solvent resistance of Pseudomonas putida DOT-T1E. J Bacteriol 181(18):5693–5700
Kabelitz N, Santos PM, Heipieper HJ (2003) Effect of aliphatic alcohols on growth and degree of saturation of membrane lipids in Acinetobacter calcoaceticus. FEMS Microbiol Lett 220(2):223–227. https://doi.org/10.1016/S0378-1097(03)00103-4
Kadurugamuwa JL, Beveridge TJ (1995) Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol 177(14):3998–4008
Kadurugamuwa JL, Beveridge TJ (1997) Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release. J Antimicrob Chemoth 40(5):615–621. https://doi.org/10.1093/jac/40.5.615
Keweloh H, Heipieper HJ (1996) Trans unsaturated fatty acids in bacteria. Lipids 31(2):129–137
Kim J, Park HD, Chung S (2012) Microfluidic approaches to bacterial biofilm formation. Molecules 17(8):9818–9834. https://doi.org/10.3390/molecules17089818
Kiran MD, Annapoorni S, Suzuki I, Murata N, Shivaji S (2005) Cis-trans isomerase gene in psychrophilic Pseudomonas syringae is constitutively expressed during growth and under conditions of temperature and solvent stress. Extremophiles 9(2):117–125. https://doi.org/10.1007/s00792-005-0435-6
Kotchaplai P, Khan E, Vangnai AS (2017) Membrane alterations in Pseudomonas putida F1 exposed to nanoscale zerovalent iron: effects of short-term and repetitive nZVI exposure. Environ Sci Technol 51(14):7804–7813. https://doi.org/10.1021/acs.est.7b00736
Kuehn MJ, Kesty NC (2005) Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev 19(22):2645–2655. https://doi.org/10.1101/gad.1299905
Kulig W, Pasenkiewicz-Gierula M, Rog T (2016) Cis and trans unsaturated phosphatidylcholine bilayers: a molecular dynamics simulation study. Chem Phys Lipids 195:12–20. https://doi.org/10.1016/j.chemphyslip.2015.07.002
Kulp A, Kuehn MJ (2010) Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol 64:163–184. https://doi.org/10.1146/annurev.micro.091208.073413
Laane C, Boeren S, Vos K, Veeger C (1987) Rules for optimization of biocatalysis in organic solvents. Biotechnol Bioeng 30(1):81–87. https://doi.org/10.1002/bit.260300112
Li Z, Clarke AJ, Beveridge TJ (1996) A major autolysin of Pseudomonas aeruginosa: subcellular distribution, potential role in cell growth and division and secretion in surface membrane vesicles. J Bacteriol 178(9):2479–2488
Loffhagen N, Hartig C, Geyer W, Voyevoda M, Harms H (2007) Competition between cis, trans and cyclopropane fatty acid formation and its impact on membrane fluidity. Eng Life Sci 7(1):67–74. https://doi.org/10.1002/elsc.200620168
Löffler C, Eberlein C, Mausezahl I, Kappelmeyer U, Heipieper HJ (2010) Physiological evidence for the presence of a cis-trans isomerase of unsaturated fatty acids in Methylococcus capsulatus bath to adapt to the presence of toxic organic compounds. FEMS Microbiol Lett 308(1):68–75. https://doi.org/10.1111/j.1574-6968.2010.01993.x
Macdonald IA, Kuehn MJ (2013) Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. J Bacteriol 195(13):2971–2981. https://doi.org/10.1128/JB.02267-12
Macdonald PM, Sykes BD, McElhaney RN (1985) Fluorine-19 nuclear magnetic resonance studies of lipid fatty acyl chain order and dynamics in Acholeplasma laidlawii B membranes. A direct comparison of the effects of cis and trans cyclopropane ring and double-bond substituents on orientational order. Biochemistry 24(17):4651–4659
Makin SA, Beveridge TJ (1996) Pseudomonas aeruginosa PAO1 ceases to express serotype-specific lipopolysaccharide at 45 degrees C. J Bacteriol 178(11):3350–3352
Manning AJ, Kuehn MJ (2011) Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol 11:258. https://doi.org/10.1186/1471-2180-11-258
Maredia R, Devineni N, Lentz P, Dallo SF, Yu J, Guentzel N, Chambers J, Arulanandam B, Haskins WE, Weitao T (2012) Vesiculation from Pseudomonas aeruginosa under SOS. Sci World J 2012:402919. https://doi.org/10.1100/2012/402919
Mashburn-Warren L, Howe J, Garidel P, Richter W, Steiniger F, Roessle M, Brandenburg K, Whiteley M (2008a) Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol Microbiol 69(2):491–502
Mashburn-Warren L, McLean RJ, Whiteley M (2008b) Gram-negative outer membrane vesicles: beyond the cell surface. Geobiology 6(3):214–219. https://doi.org/10.1111/j.1472-4669.2008.00157.x
McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ (2006) Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J Bacteriol 188(15):5385–5392. https://doi.org/10.1128/JB.00498-06
Molina-Santiago C, Udaondo Z, Gomez-Lozano M, Molin S, Ramos JL (2017) Global transcriptional response of solvent-sensitive and solvent-tolerant Pseudomonas putida strains exposed to toluene. Environ Microbiol 19(2):645–658. https://doi.org/10.1111/1462-2920.13585
Morita N, Shibahara A, Yamamoto K, Shinkai K, Kajimoto G, Okuyama H (1993) Evidence for cis-trans isomerization of a double bond in the fatty acids of the psychrophilic bacterium Vibrio sp strain Abe-1. J Bacteriol 175(3):916–918
Naether DJ, Slawtschew S, Stasik S, Engel M, Olzog M, Wick LY, Timmis KN, Heipieper HJ (2013) Adaptation of the hydrocarbonoclastic bacterium Alcanivorax borkumensis SK2 to alkanes and toxic organic compounds: a physiological and transcriptomic approach. Appl Environ Microbiol 79(14):4282–4293. https://doi.org/10.1128/AEM.00694-13
Nakae T (1976) Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun 71(3):877–884
Neumann G, Cornelissen S, van Breukelen F, Hunger S, Lippold H, Loffhagen N, Wick LY, Heipieper HJ (2006) Energetics and surface properties of Pseudomonas putida DOT-T1E in a two-phase fermentation system with 1-decanol as second phase. Appl Environ Microbiol 72(6):4232–4238. https://doi.org/10.1128/AEM.02904-05
Neumann G, Kabelitz N, Zehnsdorf A, Miltner A, Lippold H, Meyer D, Schmid A, Heipieper HJ (2005) Prediction of the adaptability of Pseudomonas putida DOT-T1E to a second phase of a solvent for economically sound two-phase biotransformations. Appl Environ Microbiol 71(11):6606–6612. https://doi.org/10.1128/AEM.71.11.6606-6612.2005
Okuyama H, Okajima N, Sasaki S, Higashi S, Murata N (1991) The cis trans isomerization of the double bond of a fatty acid as a strategy for adaptation to changes in ambient temperature in the psychrophilic bacterium, Vibrio sp strain Abe-1. Biochim Biophys Acta 1084(1):13–20. https://doi.org/10.1016/0005-2760(91)90049-N
Okuyama H, Ueno A, Enari D, Morita N, Kusano T (1998) Purification and characterization of 9-hexadecenoic acid cis-trans isomerase from Pseudomonas sp strain E-3. Arch Microbiol 169(1):29–35
Pedrotta V, Witholt B (1999) Isolation and characterization of the cis-trans-unsaturated fatty acid isomerase of Pseudomonas oleovorans GPo12. J Bacteriol 181(10):3256–3261
Pierce BK, Voegel T, Kirkpatrick BC (2014) The Xylella fastidiosa PD1063 protein is secreted in association with outer membrane vesicles. PLoS One 9(11):e113504. https://doi.org/10.1371/journal.pone.0113504
Pinkart HC, Wolfram JW, Rogers R, White DC (1996) Cell envelope changes in solvent-tolerant and solvent-sensitive Pseudomonas putida strains following exposure to o-xylene. Appl Environ Microbiol 62(3):1129–1132
Piotrowska A, Syguda A, Chrzanowski L, Heipieper HJ (2016) Toxicity of synthetic herbicides containing 2,4-D and MCPA moieties towards Pseudomonas putida mt-2 and its response at the level of membrane fatty acid composition. Chemosphere 144:107–112. https://doi.org/10.1016/j.chemosphere.2015.08.067
Ramos JL, Gallegos MT, Marques S, Ramos-Gonzalez MI, Espinosa-Urgel M, Segura A (2001) Responses of gram-negative bacteria to certain environmental stressors. Curr Opin Microbiol 4(2):166–171. https://doi.org/10.1016/S1369-5274(00)00183-1
Roach C, Feller SE, Ward JA, Shaikh SR, Zerouga M, Stillwell W (2004) Comparison of cis and trans fatty acid containing phosphatidylcholines on membrane properties. Biochemistry 43(20):6344–6351. https://doi.org/10.1021/bi049917r
Sabra W, Lunsdorf H, Zeng AP (2003) Alterations in the formation of lipopolysaccharide and membrane vesicles on the surface of Pseudomonas aeruginosa PAO1 under oxygen stress conditions. Microbiology 149:2789–2795. https://doi.org/10.1099/mic.0.26443-0
Schertzer JW, Boulette ML, Whiteley M (2009) More than a signal: non-signaling properties of quorum sensing molecules. Trends Microbiol 17(5):189–195. https://doi.org/10.1016/j.tim.2009.02.001
Schertzer JW, Brown SA, Whiteley M (2010) Oxygen levels rapidly modulate Pseudomonas aeruginosa social behaviours via substrate limitation of PqsH. Mol Microbiol 77(6):1527–1538. https://doi.org/10.1111/j.1365-2958.2010.07303.x
Schertzer JW, Whiteley M (2012) A bilayer-couple model of bacterial outer membrane vesicle biogenesis. MBio 3(2):e00297–e00211. https://doi.org/10.1128/mBio.00297-11
Schertzer JW, Whiteley M (2013) Bacterial outer membrane vesicles in trafficking, communication and the host-pathogen interaction. J Mol Microbiol Biotechnol 23(1–2):118–130. https://doi.org/10.1159/000346770
Schooling SR, Beveridge TJ (2006) Membrane vesicles: an overlooked component of the matrices of biofilms. J Bacteriol 188(16):5945–5957. https://doi.org/10.1128/Jb.00257-06
Schwechheimer C, Kuehn MJ (2013) Synthetic effect between envelope stress and lack of outer membrane vesicle production in Escherichia coli. J Bacteriol 195(18):4161–4173. https://doi.org/10.1128/Jb.02192-12
Schwechheimer C, Kuehn MJ (2015) Outer-membrane vesicles from gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol 13(10):605–619. https://doi.org/10.1038/nrmicro3525
Schwechheimer C, Sullivan CJ, Kuehn MJ (2013) Envelope control of outer membrane vesicle production in gram-negative bacteria. Biochemistry 52(18):3031–3040. https://doi.org/10.1021/bi400164t
Seelig J, Waespe-Sarcevic N (1978) Molecular order in cis and trans unsaturated phospholipid bilayers. Biochemistry 17(16):3310–3315. https://doi.org/10.1021/bi00609a021
Segura A, Duque E, Mosqueda G, Ramos JL, Junker F (1999) Multiple responses of gram-negative bacteria to organic solvents. Environ Microbiol 1(3):191–198
Sikkema J, de Bont JAM, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59(2):201–222
Sinensky M (1974) Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A 71(2):522–525
Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209. https://doi.org/10.1146/annurev.micro.56.012302.160705
Sutherland IW (2001) The biofilm matrix—an immobilized but dynamic microbial environment. Trends Microbiol 9(5):222–227. https://doi.org/10.1016/S0966-842x(01)02012-1
Suutari M, Laakso S (1994) Microbial fatty acids and thermal adaptation. Crit Rev Microbiol 20(4):285–328. https://doi.org/10.3109/10408419409113560
Tan ZG, Yoon JM, Nielsen DR, Shanks JV, Jarboe LR (2016) Membrane engineering via trans unsaturated fatty acids production improves Escherichia coli robustness and production of biorenewables. Metab Eng 35:105–113. https://doi.org/10.1016/j.ymben.2016.02.004
van den Berg B (2012) Structural basis for outer membrane sugar uptake in pseudomonads. J Biol Chem 287(49):41044–41052. https://doi.org/10.1074/jbc.M112.408518
van Loosdrecht MC, Lyklema J, Norde W, Schraa G, Zehnder AJ (1987) The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol 53(8):1893–1897
von Wallbrunn A, Richnow HH, Neumann G, Meinhardt F, Heipieper HJ (2003) Mechanism of cis-trans isomerization of unsaturated fatty acids in Pseudomonas putida. J Bacteriol 185(5):1730–1733
Weber FJ, de Bont JAM (1996) Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim Biophys Acta 1286(3):225–245
Weitere M, Bergfeld T, Rice SA, Matz C, Kjelleberg S (2005) Grazing resistance of Pseudomonas aeruginosa biofilms depends on type of protective mechanism, developmental stage and protozoan feeding mode. Environ Microbiol 7(10):1593–1601. https://doi.org/10.1111/j.1462-2920.2005.00851.x
Wessel AK, Liew J, Kwon T, Marcotte EM, Whiteley M (2013) Role of Pseudomonas aeruginosa peptidoglycan-associated outer membrane proteins in vesicle formation. J Bacteriol 195(2):213–219. https://doi.org/10.1128/Jb.01253-12
Wick LY, Pasche N, Bernasconi SM, Pelz O, Harms H (2003) Characterization of multiple-substrate utilization by anthracene-degrading Mycobacterium frederiksbergense LB501T. Appl Environ Microbiol 69(10):6133–6142
Wick LY, Ruiz de Munain A, Springael D, Harms H (2002) Responses of Mycobacterium sp. LB501T to the low bioavailability of solid anthracene. Appl Microbiol Biotechnol 58(3):378–385. https://doi.org/10.1007/s00253-001-0898-z
Zhang YM, Rock CO (2008) Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6(3):222–233. https://doi.org/10.1038/nrmicro1839
Zhou L, Srisatjaluk R, Justus DE, Doyle RJ (1998) On the origin of membrane vesicles in gram-negative bacteria. FEMS Microbiol Lett 163(2):223–228
Acknowledgements
The financial support by the European Union’s Horizon 2020 research and innovation program under grant agreement no. 633962 for the project P4SB is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Eberlein, C., Baumgarten, T., Starke, S. et al. Immediate response mechanisms of Gram-negative solvent-tolerant bacteria to cope with environmental stress: cis-trans isomerization of unsaturated fatty acids and outer membrane vesicle secretion. Appl Microbiol Biotechnol 102, 2583–2593 (2018). https://doi.org/10.1007/s00253-018-8832-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8832-9