Abstract
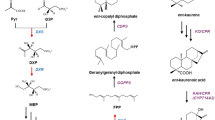
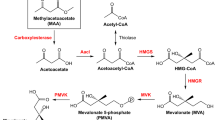
Cycloartenol is biosynthetically the first sterol skeleton, which is metabolized to phytosterols such as β-sitosterol and stigmasterol. β-Amyrin is the most commonly occurring aglycone skeleton for oleanane-type saponins such as glycyrrhizin and saikosaponins. It has been regarded that these cyclic triterpenes are unable to be produced in Escherichia coli, while no reports are available on their production with E. coli. Here, we describe a method to synthesize triterpene skeletons from higher plants, including cycloartenol and β-amyrin. We introduced into E. coli the biosynthetic pathway genes from farnesyl diphosphate (FPP) to cycloartenol or β-amyrin, which contained Arabidopsis (Arabidopsis thaliana)-derived squalene synthase (AtSQS) and squalene epoxidase (AtSQE) genes in addition to the Arabidopsis cycloartenol synthase (AtCAS1) gene, or the β-amyrin synthase (EtAS) gene of the petroleum plant Euphorbia tirucalli, along with the isopentenyl diphosphate isomerase (HpIDI) gene from a green algae Haematococcus pluvialis. The order of genes, HpIDI, AtSQS, AtSQE, driven by transcriptional read-through from a tac promoter to an rrnB terminator, was crucial for their functional expression in E. coli to produce cycloartenol or β-amyrin. The co-expression of a bacterial NADPH-regenerating gene (zwf or gdh) as well as bacterial redox partner protein genes (camA and camB, or NsRED and NsFER) was found to increase the amounts of these triterpenes several fold. The present study could open up opportunities not only to carry out functional analysis of a higher-plant-derived oxidosqualene cyclase (OSC) gene in E. coli but also to produce functional triterpenes that originate from medicinal or herbal plants.







Similar content being viewed by others
References
Agematu H, Matsumoto N, Fujii Y, Kabumoto H, Doi S, Machida K, Ishikawa J, Arisawa A (2006) Hydroxylation of testosterone by bacterial cytochromes P450 using the Escherichia coli expression system. Biosci Biotechnol Biochem 70:307–311
Agger SA, Lopez-Gallego F, Hoye TR, Schmidt-Dannert C (2008) Identification of sesquiterpene synthases from Nostoc punctiforme PCC 73102 and Nostoc sp. strain PCC7120. J Bacteriol 190:6084–6096
Ajikumar PK, Tyo K, Carlsen S, Mucha O, Phon TH, Stephanopoulos G (2008) Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol Pharm 5:167–190
Ajikumar PK, Xiao WH, Tyo KEJ, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G (2010) Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli. Science 330:70–74
Araki M, Kaku N, Harada M, Ando Y, Yamaguchi R, Shindo K (2016) Production of auroxanthins from violaxanthin and 9-cis-violaxanthin by acidic treatment and the antioxidant activities of violaxanthin, 9-cis-violaxanthin, and auroxanthins. J Agric Food Chem 64:9352–9355
Augustin JM, Kuzina V, Andersen SB, Bak S (2011) Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 72:435–457
Carter OA, Peters RJ, Croteau R (2003) Monoterpene biosynthesis pathway construction in Escherichia coli. Phytochemistry 64:425–433
Degenhardt J, Köllner TG, Gershenzon J (2009) Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 70:1621–1637
Dewick PM (2009) Medicinal natural products a biosynthetic approach Third Edition. A Wiley
Engels B, Dahm P, Jennewein S (2008) Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (paclitaxel) production. Met Eng 10:201–206
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Fujisaki S, Hara H, Nishizima Y, Horiuchi K, Nishino T (1990) Cloning and nucleotide sequence of the ispA gene responsible for farnesyl diphosphate synthase activity in Escherichia coli. J Biochem 108:995–1000
Fujita N, Sumisa F, Shindo K, Kabumoto H, Arisawa A, Ikenaga H, Misawa N (2009) Comparison of two vectors for functional expression of a bacterial cytochrome P450 gene in Escherichia coli using CYP153 genes. Biosci Biotechnol Biochem 73:1825–1830
Ghimire GP, Lee HC, Sohng JK (2009) Improved squalene production via modulation of the methylerythritol 4-phophate pathway and heterologous expression of genes from Streptomyces peucetius ATCC 27952 in Escherichia coli. Appl Environ Microbiol 75:7291–7293
Girhard M, Machida K, Itoh M, Schmid RD, Arisawa A, Urlacher VB (2009) Regioselective biooxidation of (+)-valencene by recombinant E. coli expressing CYP109B1 from Bacillus subtilis in a two-liquid-phase system. Microb Cell Factories 8:36
Ginglinger JF, Boachon B, Hofer R, Paetz C, Kollner TG, Miesch L, Lugan R, Baltenweck R, Mutterer J, Ullmann P, Beran F, Claudel P, Verstappen F, Fischer MJ, Karst F, Bouwmeester H, Miesch M, Schneider B, Gershenzon J, Ehlting J, Werck-Reichhart D (2013) Gene coexpression analysis reveals complex metabolism of the monoterpene alcohol linalool in Arabidopsis flowers. Plant Cell 25:4640–4657
Harada H, Misawa N (2009) Novel approaches and achievements in biosynthesis of functional isoprenoids in Escherichia coli. Appl Microbiol Biotechnol 84:1021–1031
Harada H, Shindo K, Iki K, Teraoka A, Okamoto S, Yu F, Hattan J, Utsumi R, Misawa N (2011) Efficient functional analysis system for cyanobacterial or plant cytochromes P450 involved in sesquiterpene biosynthesis. Appl Microbiol Biotechnol 90:467–476
Harada H, Yu F, Okamoto S, Kuzuyama T, Utsumi R, Misawa N (2009) Efficient synthesis of functional isoprenoids from acetoacetate through metabolic pathway-engineered Escherichia coli. Appl Microbiol Biotechnol 81:915–925
Husselstein-Muller T, Schaller H, Benveniste P (2001) Molecular cloning and expression in yeast of 2,3-oxidosqualene-triterpenoid cyclases from Arabidopsis thaliana. Plant Mol Biol 45:75–92
Immethun CM, Hoynes-O’Connor AG, Balassy A, Moon TS (2013) Microbial production of isoprenoids enabled by synthetic biology. Front Microbiol 4:75 (1-8)
Jackson H, Braun CL, Ernst H (2008) The chemistry of novel xanthophyll carotenoids. Am J Cardiol 101:50D–57D
Jiang M, Stephanopoulos G, Pfeifer BA (2012) Toward biosynthetic design and implementation of Escherichia coli-derived paclitaxel and other heterologous polyisoprene compounds. Appl Environ Microbiol 78:2497–2504
Kajikawa M, Yamato KT, Fukuzawa H, Sakai Y, Uchida H, Ohyama K (2005) Cloning and characterization of cDNA encoding β-amyrin synthase from petroleum plant Euphorbia tirucalli L. Phytochemistry 66:1759–1766
Kajiwara S, Fraser PD, Kondo K, Misawa N (1997) Expression of an exogenous isopentenyl diphosphate isomerase gene enhances isoprenoid biosynthesis in Escherichia coli. Biochem J 324:421–426
Kannenberg EL, Poralla K (1999) Hopanoid biosynthesis and function in bacteria. Naturwissenschaften 86:168–176
Katabami A, Li L, Iwasaki M, Furubayashi M, Saito K. Umeno D (2015) Production of squalene by squalene synthases and their truncated mutants in Escherichia coli. J Biosci Bioeng 119:165–171
Kirby J, Keasling JD (2009) Biosynthesis of plant isoprenoids: perspectives for microbial engineering. Annu Rev Plant Biol 60:335–355
Kirby J, Nishimoto M, Chow RWN, Baidoo EEK, Wang G, Martin J, Schackwitz W, Chan R, Fortman JL, Keasling JD (2015) Enhancing terpene yield from sugars via novel routes to 1-deoxy-D-xylulose 5-phosphate. Appl Environ Microbiol 81:130–138
Kirby J, Romanini DW, Paradise EM, Keasling JD (2008) Engineering triterpene production in Saccharomyces cerevsiae -β-amyrin synthase from Artemisia annua. FEBS J 275:1852–1859
Kurokawa H, Koyama T (2010) Prenyltransferase. In: Mander L, Lui H-W (eds) Comprehensive natural products II Chemistry and biology, vol volume 1. Elsevier, Oxford, pp 557–584
Kusama H, Hara R, Kawahara S, Nishimori T, Kashima H, Nakamura N, Morihira K, Kuwajima I (2000) Enantioselective total synthesis of (−)-taxol. J Am Chem Soc 122:3811–3820
Kushiro T, Ebizuka Y (2010) Triterpenes. In: Mander L, Lui H-W (eds) Comprehensive natural products II Chemistry and biology, vol volume 1. Elsevier, Oxford, pp 673–708
Kushiro T, Shibuya M, Ebizuka Y (1998) β-Amyrin synthase cloning of oxidosqualene cyclase that catalyzes the formation of the most popular triterpene among higher plants. Eur J Biochem 256:238–244
Lemuth K, Steuer K, Albermann C (2011) Engineering of a plasmid-free Escherichia coli strain for improved in vivo biosynthesis of astaxanthin. Microb Cell Factories 10:29
Li D, Zhang Q, Zhou Z, Zhao F, Lu W (2016) Heterologous biosynthesis of triterpenoid dammarenediol-II in engineered Escherichia coli. Biotechnol Lett 38:603–609
Misawa N (2010) Carotenoids. In: Mander L, Lui H-W (eds) Comprehensive natural products II Chemistry and biology, vol volume 1. Elsevier, Oxford, pp 733–753
Misawa N (2011) Pathway engineering for functional isoprenoids. Curr Opin Biotechnol 22:627–633
Misawa N, Shimada S (1998) Metabolic engineering for the production of carotenoids in non-carotenogenic bacteria and yeasts. J Biotechnol 59:169–181
Morrone D, Lowry L, Determan MK, Hershey DM, Xu M, Peters RJ (2010) Increasing diterpene yield with a modular metabolic engineering system in E. coli: comparison of MEV and MEP isoprenoid precursor pathway engineering. Appl Microbiol Biotechnol 85:1893–1906
Muntendam R, Melillo E, Ryden A, Kayser O (2009) Perspectives and limits of engineering the isoprenoid metabolism in heterologous host. Appl Microbiol Biotechnol 84:1003–1019
Nagumo A, Kamei T, Sakakibara J, Ono T (1995) Purification and characterization of recombinant squalene epoxidase. J Lipid Res 36:1489–1497
Nakano C, Motegi A, Sato T, Onodera M, Hoshino T (2007) Sterol biosynthesis by a prokaryote: first in vitro identification of the genes encoding squalene epoxidase and lanosterol synthase from Methylococcus capsulatus. Bisci Biotechnol Biochem 71:2543–2550
Rico J, Pardo E, Orejas M (2010) Enhanced production of a plant monoterpene by overexpression of the 3-hydroxy-3-methylglutaryl coenzyme A reductase catalytic domain in Saccharomyces cerevisiae. Appl Environ Microbiol 76:6449–6454
Roberts SC (2007) Production and engineering of terpenoids in plant cell culture. Nat Chem Biol 3:387–395
Rose RE (1988) The nucleotide sequence of pACYC184. Nucleic Acids Res 16:355
Sakakibara J, Watanabe R, Kanai Y, Ono T (1995) Molecular cloning and expression of rat squalene epoxidase. J Biol Chem 270:17–20
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual
Sandmann G (2002) Combinatorial biosynthesis of carotenoids in a heterologous host: a powerful approach for the biosynthesis of novel structures. Chem Bio Chem 3:629–635
Sawai S, Shindo T, Sato S, Kaneko T, Tabata S, Ayabe S, Aoki T (2006) Functional and structural analysis of genes encoding oxidosqualene cyclases of Lotus japonicus. Plant Sci 170:247–257
Schewe H, Holtmann D, Schrader J (2009) P450BM-3-catalyzed whole-cell biotransformation of α-pinene with recombinant Escherichia coli in an aqueous-organic two-phase system. Appl Microbiol Biotechnol 83:849–857
Seki H, Ohyama K, Sawai S, Mizutani M, Ohnishi T, Sudo H, Akashi T, Aoki T, Saito K, Muranaka T (2008) Licorice β-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc Natl Acad Sci U S A 105:14204–14209
Seki H, Sawai S, Ohyama K, Mizutani M, Ohnishi T, Sudo H, Fukushima EO, Akashi T, Aoki T, Saito K, Muranaka T (2011) Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin. Plant Cell 23:4112–4123
Sun Y, Sun L, Shang F, Yan G (2016) Enhanced production of β-carotene in recombinant Saccharomyces cerevisiae by inverse metabolic engineering with supplementation of unsaturated fatty acids. Process Biochem 51:568–577
Suzuki S, Koeduka T, Sugiyama A, Yazaki K, Umezawa T (2014) Microbial production of plant specialized metabolites. Plant Biotechnol 31:465–482
Suzuki M, Xiang T, Ohyama K, Seki H, Saito K, Muranaka T, Hayashi H, Katsube Y, Kushiro T, Shibuya M, Ebizuka Y (2006) Lanosterol synthase in dicotyledonous plants. Plant Cell Physiol 47:565–571
Takemura M, Maoka M, Misawa N (2015) Biosynthetic routes of hydroxylated carotenoids (xanthophylls) in Marchantia polymorpha, and production of novel and rare xanthophylls through pathway engineering in Escherichia coli. Planta 241:699–710
Tansakul P, Shibuya M, Kushiro T, Ebizuka Y (2006) Dammarenediol II synthase, the first dedicated enzyme for ginsenoside biosynthesis, in Panax ginseng. FEBS Lett 580:5143–5149
Wu S, Schalk M, Clark A, Miles RB, Coates R, Chappell J (2006) Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nat Biotechnol 24:1441–1447
Yamano S, Ishii T, Nakagawa M, Ikenaga H, Misawa N (1994) Metabolic engineering for production of β-carotene and lycopene in Saccharomyces cerevisiae. Biosci Biotechnol Biochem 58:1112–1114
Acknowledgements
The authors thank Mr. Toshihiko Otomatsu, KNC Laboratories Co., Ltd., for the gift of plasmid pET-SoFNR/FER.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Takemura, M., Tanaka, R. & Misawa, N. Pathway engineering for the production of β-amyrin and cycloartenol in Escherichia coli—a method to biosynthesize plant-derived triterpene skeletons in E. coli . Appl Microbiol Biotechnol 101, 6615–6625 (2017). https://doi.org/10.1007/s00253-017-8409-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8409-z