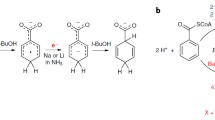
Abstract
The bacterial dioxygenation of mono- or polycyclic aromatic compounds is an intensely studied field. However, only in a few cases has the repeated dioxygenation of a substrate possessing more than a single aromatic ring been described. We previously characterized the aryl-hydroxylating dioxygenase BphA-B4h, an artificial hybrid of the dioxygenases of the biphenyl degraders Burkholderia xenovorans LB400 and Pseudomonas sp. strain B4-Magdeburg, which contains the active site of the latter enzyme, as an exceptionally powerful biocatalyst. We now show that this dioxygenase possesses a remarkable capacity for the double dioxygenation of various bicyclic aromatic compounds, provided that they are carbocyclic. Two groups of biphenyl analogues were examined: series A compounds containing one heterocyclic aromatic ring and series B compounds containing two homocyclic aromatic rings. Whereas all of the seven partially heterocyclic biphenyl analogues were solely dioxygenated in the homocyclic ring, four of the six carbocyclic bis-aryls were converted into ortho,meta-hydroxylated bis-dihydrodiols. Potential reasons for failure of heterocyclic dioxygenations are discussed. The obtained bis-dihydrodiols may, as we also show here, be enzymatically re-aromatized to yield the corresponding tetraphenols. This opens a way to a range of new polyphenolic products, a class of compounds known to exert multiple biological activities. Several of the obtained compounds are novel molecules.


Similar content being viewed by others
References
Barry SM, Challis GL (2013) Mechanism and catalytic diversity of Rieske non-heme iron-dependent oxygenases. ACS Catal 3:2362–2370
Birudukota NVS, Franke R, Hofer B (2016) An approach to “Escape from flatland”: chemo-enzymatic synthesis and biological profiling of a library of bridged bicyclic compounds. Org Biomol Chem. doi:10.1039/C5OB02539G
Boyd DR, McMordie RAS, Porter HP, Dalton H, Jenkins RO, Howarth OW (1987) Metabolism of bicyclic aza-arenes by Pseudomonas putida to yield vicinal cis-dihydrodiols and phenols. J Chem Soc Chem Comm 22:1722–1724
Boyd DR, Sharma ND, Belhocine T, Malone JF, McGregor S, Allen CCR (2006) Dioxygenase-catalysed dihydroxylation of arene cis-dihydrodiols and acetonide derivatives: a new approach to the synthesis of enantiopure tetraoxygenated bioproducts from arenes. Chem. Commun. (Camb.) 4934–4936
Boyd DR, Sharma ND, Brannigan IN, Haughey SA, Malone JF, Clarke DA, Dalton H (1996) Dioxygenase-catalysed formation of cis/trans-dihydrodiol metabolites of mono-and bi-cyclic heteroarenes. Chem. Commun. (Camb.) 2361–2362
Boyd DR, Sharma ND, Carroll JG, Malone JF, Mackerracher DG, Allen CCR (1998) Dioxygenase-catalysed cis-dihydrodiol formation in the carbo- and hetero-cyclic rings xof quinolines. Chem. Commun. (Camb.) 683–684
Boyd DR, Sharma ND, Coen GP, Hempenstall F, Ljubez V, Malone JF, Allen CCR, Hamilton JTG (2008) Regioselectivity and stereoselectivity of dioxygenase catalysed cis-dihydroxylation of mono- and tri-cyclic azaarene substrates. Org Biomol Chem 6:3957–3966
Boyd DR, Sharma ND, Dorrity MRJ, Hand MV, McMordie RAS, Malone JF, Porter HO, Dalton H, Chima H, Sheldrake GN (1993) Structure and stereochemistry of cis-dihydrodiol and phenol metabolites of bicyclic azaarenes from Pseudomonas putida UV4. J Chem Soc Perkin Trans 1 9:1065–1071
Boyd DR, Sharma ND, Hempenstall F, Kennedy MA, Malone JF, Allen CCR, Resnick SM, Gibson DT (1999) bis-cis-Dihydrodiols: a new class of metabolites resulting from biphenyl dioxygenase-catalyzed sequential asymmetric cis-dihydroxylation of polycyclic arenes and heteroarenes. J Org Chem 64:4005–4011
Boyd DR, Sheldrake GN (1998) The dioxygenase-catalyzed formation of vicinal cis-diols. Nat Prod Rep 15:309–325
Bugg TD, Ramaswamy S (2008) Non-heme iron-dependent dioxygenases: unravelling catalytic mechanisms for complex enzymatic oxidations. Curr Opin Chem Biol 12:134–140
Cámara B, Seeger M, González M, Standfuß-Gabisch C, Kahl S, Hofer B (2007) Generation by a widely applicable approach of a hybrid dioxygenase showing improved oxidation of polychlorobiphenyls. Appl Environ Microbiol 73:2682–2689
Chemical Abstracts Services (2015) SciFinder database www.cas.org/products/scifinder
Ensley BD, Ratzkin BJ, Osslund TD, Simon MJ, Wackett LP, Gibson DT (1983) Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science 222:167–169
Fetzner S (2000) Enzymes involved in the aerobic bacterial degradation of N-heteroaromatic compounds: molybdenum hydroxylases and ring-opening 2,4-dioxygenases. Naturwissenschaften 87:59–69
Furukawa K (2000) Biochemical and genetic bases of microbial degradation of polychlorinated biphenyls (PCBs). J Gen Appl Microbiol 46:283–296
Furukawa K, Hirose J, Suyama A, Zainki T, Hayashida S (1993) Gene components responsible for discrete substrate specificity in the metabolism of biphenyl (bph operon) and toluene (tod operon). J Bacteriol 175:5224–5232
Garrett MD, Scott R, Sheldrake GN, Dalton H, Goode P (2006) Biotransformation of substituted pyridines with dioxygenase-containing microorganisms. Org Biomol Chem 4:2710–2715
Hille R (2005) Molybdenum-containing hydroxylases. Arch Biochem Biophys 433:107–116
Haddock JD, Horton JR, Gibson DT (1995) Dihydroxylation and dechlorination of chlorinated biphenyls by purified biphenyl 2,3-dioxygenase from Pseudomonas sp. strain LB400. J Bacteriol 177:20–26
Hudlicky T, Gonzalez D, Gibson DT (1999) Enzymatic hydroxylation of aromatics in enantioselective synthesis: expanding asymmetric methodology. Aldrichim Acta 32:35–62
Hudlicky T, Reed J (2009) Celebrating 20 years of Synlett—special account on the merits of biocatalysis and the impact of arene cis-dihydrodiols on enantioselective synthesis. Synlett 2009:685–703
Johnson RA (2004) Microbial arene oxidations. Org React 63:117–264
Kahl S, Hofer B (2003) A genetic system for the rapid isolation of aromatic-ring-hydroxylating dioxygenase activities. Microbiology 149:1475–1481
Kimura N, Kato H, Nishi A, Furukawa K (1996) Analysis of substrate range of biphenyl-catabolic enzymes. Biosci Biotechnol Biochem 60:220–223
Kobayashi S, Kuno S, Itada N, Hayaishi O, Kozuka S, Oae S (1964) O-18 studies on anthranilate hydroxylase—a novel mechanism of double hydroxylation. Biochem Biophys Res Commun 16:556–561
Misawa N, Shindo K, Takahashi H, Suenaga H, Iguchi K, Okazaki H, Harayama S, Furukawa K (2002) Hydroxylation of various molecules including heterocyclic aromatics using recombinant Escherichia coli cells expressing modified biphenyl dioxygenase genes. Tetrahedron 58:9605–9612
Mushtaq M, Wani SM (2013) Polyphenols and human health—a review. Int J Pharm Bio Sci 4:B338–B360
Overwin H, González M, Méndez V, Cárdenas F, Seeger M, Hofer B (2015a) Stepwise conversion of flavonoids by engineered dioxygenases and dehydrogenase: characterization of novel biotransformation products. Enzyme Microb Tech 81:63–71
Overwin H, González M, Méndez V, Seeger M, Wray V, Hofer B (2012) Dioxygenation of the biphenyl dioxygenation product. Appl Environ Microbiol 78:4529–4532
Overwin H, Standfuß-Gabisch C, González M, Méndez V, Seeger M, Reichelt J, Wray V, Hofer B (2015b) Permissivity of the biphenyl-specific aerobic bacterial metabolic pathway towards analogues with various steric requirements. Microbiology 161:1844–1856
Pasinetti GM, Wang J, Ho L, Zhao W, Dubner L (2015) Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim Biophys Acta 1852:1202–1208
Pazos M, Martínez S, Vila MA, Rodríguez P, Veiga N, Seoane G, Carrera I (2015) Aza and oxo Diels–Alder reactions using cis-cyclohexadienediols of microbial origin: chemoenzymatic preparation of synthetically valuable heterocyclic scaffolds. Tetrahedron-Asymmet 26:1436–1447
Rivard BS, Rogers MS, Marell DJ, Neibergall MB, Chakrabarty S, Cramer CJ, Lipscomb JD (2015) Rate-determining attack on substrate precedes Rieske cluster oxidation during cis-dihydroxylation by benzoate dioxygenase. Biochemistry 54:4652–4664
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor, Cold Spring Harbor Laboratory
Seeger M, Timmis KN, Hofer B (1995) Degradation of chlorobiphenyls catalyzed by the bph-encoded biphenyl-2,3-dioxygenase and biphenyl-2,3-dihydrodiol-2,3-dehydrogenase of Pseudomonas sp. LB400. FEMS Microbiol Lett 133:259–264
Seeger M, Zielinski M, Timmis KN, Hofer B (1999) Regiospecificity of dioxygenation of di- to pentachlorobiphenyls and their degradation to chlorobenzoates by the bph-encoded catabolic pathway of Burkholderia sp. strain LB400. Appl Environ Microbiol 65:3614–3621
Shindo K, Shindo Y, Hasegawa T, Osawa A, Kagami O, Furukawa K, Misawa N (2007) Synthesis of highly hydroxylated aromatics by evolved biphenyl dioxygenase and subsequent dihydrodiol dehydrogenase. Appl Microbiol Biotechnol 75:1063–1069
Studier FW (1991) Use of bacteriophage T7 lyzozyme to improve an inducible T7 expression system. J Mol Biol 219:37–44
Zielinski M, Kahl S, Standfuß-Gabisch C, Cámara B, Seeger M, Hofer B (2006) Generation of novel-substrate-accepting biphenyl dioxygenases through segmental random mutagenesis and identification of residues involved in enzyme specificity. Appl Environ Microbiol 72:2191–2199
Acknowledgments
The authors wish to thank Enno Michaelis, Yasmin Wenzel, Anne Heidelmann and Sandra Berger for help with DHD preparations, Ulrike Beutling for LC-MS analyses, Christel Kakoschke for NMR measurements and Andrea Abrahamik, Anja Meier and Manfred Nimtz for ESI-HRMS analyses. We also gratefully acknowledge financial support from CONICYT-BMBF (2009-174, 2011-642, 01DN12108). MS also acknowledges support through CONICYT 21120887 PhD and GO fellowships (to VM), FONDECYT (1110992, 1151174), USM (131109, 131342 and 131562) and CN&BS grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 180 kb)
Rights and permissions
About this article
Cite this article
Overwin, H., González, M., Méndez, V. et al. An aryl dioxygenase shows remarkable double dioxygenation capacity for diverse bis-aryl compounds, provided they are carbocyclic. Appl Microbiol Biotechnol 100, 8053–8061 (2016). https://doi.org/10.1007/s00253-016-7570-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7570-0