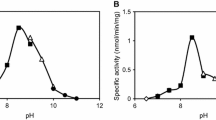
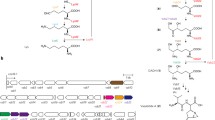
Abstract
Recent work has shed light on the abundance and diversity of d-amino acids in bacterial extracellular/periplasmic molecules, bacterial cell culture, and bacteria-rich environments. Within the extracellular/periplasmic space, d-amino acids are necessary components of peptidoglycan, and disruption of their synthesis leads to cell death. As such, enzymes responsible for d-amino acid synthesis are promising targets for antibacterial compounds. Further, bacteria are shown to incorporate a diverse collection of d-amino acids into their peptidoglycan, and differences in d-amino acid incorporation may occur in response to differences in growth conditions. Certain d-amino acids can accumulate to millimolar levels in cell culture, and their synthesis is proposed to foretell movement from exponential growth phase into stationary phase. While enzymes responsible for synthesis of d-amino acids necessary for peptidoglycan (d-alanine and d-glutamate) have been characterized from a number of different bacteria, the d-amino acid synthesis enzymes characterized to date cannot account for the diversity of d-amino acids identified in bacteria or bacteria-rich environments. Free d-amino acids are synthesized by racemization or epimerization at the α-carbon of the corresponding l-amino acid by amino acid racemase or amino acid epimerase enzymes. Additionally, d-amino acids can be synthesized by stereospecific amination of α-ketoacids. Below, we review the roles of d-amino acids in bacterial physiology and biotechnology, and we describe the known mechanisms by which they are synthesized by bacteria.



Similar content being viewed by others
References
Adams E (1959) Hydroxyproline metabolism I. Converstion to α-ketoglutarate by extracts of Pseudomonas. J Biol Chem 234:2073–2084
Arias CA, Martín-Martinez M, Blundell TL, Arthur M, Courvalin P, Reynolds PE (1999) Characterization and modelling of VanT: a novel, membrane-bound, serine racemase from vancomycin-resistant Enterococcus gallinarum BM4174. Mol Microbiol 31:1653–1664
Arias CA, Weisner J, Blackburn JM, Reynolds PE (2000) Serine and alanine racemase activities of VanT: a protein necessary for vancomycin resistance in Enterococcus gallinarum BM4174. Microbiology 146:1727–1734
Arias CA, Peña J, Panesso D, Reynolds P (2003) Role of the transmembrane domain of the VanT serine racemase in resistance to vancomycin in Enterococcus gallinarum BM4174. J Antimicrob Chemother 51:557–564
Ayengar P, Roberts E (1952) Utilization of d-glutamic acid by Lactobacillus arabinosus: glutamic racemase. J Biol Chem 197:453–460
Balibar CJ, Vaillancourt FH, Walsh CT (2005) Generation of d-amino acid residues in assembly of arthrofactin by dual condensation/epimerization domains. Chem Biol 12:1189–1200
Baltz R, Miao V, Wrigley S (2005) Natural products to drug: daptomycin and related lipopeptide antibiotics. Nat Prod Rep 22:717–741
Barreteau H, Kovač A, Boniface A, Sova M, Gobec S, Blanot D (2008) Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol Rev 32:168–207
Bella J, Eaton M, Brodsky B, Berman H (1994) Crystal and molecular structure of a collagen-like peptide at 1.9Å resolution. Science 266:75–81
Bellais S, Arthur M, Dubost L, Hugonnet J-E, Gutmann L, van Heijenoort J, Legrand R, Brouard J-P, Rice L, Mainardi J-L (2006) Aslfm, the d-aspartate ligase responsible for the addition of d-aspartic acid onto the peptidoglycan precursor of Enterococcus faecium. J Biol Chem 281:11586–11594
Boniface A, Bouhss A, Mengin-Lecreulx D, Blanot D (2006) The MurE synthetase from Thermotoga maritima is endowed with an unusual d-lysine adding activity. J Biol Chem 281:15680–15686
Boniface A, Parquet C, Arthur M, Mengin-Lecreulx D, Blanot D (2009) The elucidation of the structure of Thermotoga maritima peptidoglycan reveals two novel types of cross-link. J Biol Chem 284:21856–21862
Brückner H, Hausch M (1989) Gas chromatographic detection of d-amino acids as common constituents of fermented foods. Chromatographia 28:487–492
Brückner H, Schieber A (2001) Ascertainment of d-amino acids in germ-free, gnotobiotic and normal laboratory rats. Biomed Chromatogr 15:257–262
Buschiazzo A, Goytia M, Schaeffer F, Degrave W, Shepard W, Grégoire C, Chamond N, Cosson A, Berneman A, Coatnoan N, Alzari PM, Minoprio P (2006) Crystal structure, catalytic mechanism, and mitogenic properties of Trypanosoma cruzi proline racemase. Proc Natl Acad Sci U S A 103:1705–1710
Caboche S, Pupin M, Leclère V, Fontaine A, Jacques P, Kucherov G (2008) NORINE: a database of nonribosomal peptides. Nucleic Acids Res 36:D326–D331
Caboche S, Leclère V, Pupin M, Kucherov G, Jacques P (2010) Diversity of monomers in nonribosomal peptides: towards the prediction of origin and biological activity. J Bacteriol 192:5143–5150
Candela T, Fouet A (2006) Poly-gamma-glutamate in bacteria. Mol Microbiol 60:1091–1098
Cassab GI (1998) Plant cell wall proteins. Annu Rev Plant Physiol Plant Mol Biol 49:281–309
Cava F, de Pedro MA, Lam H, Davis BM, Waldor MK (2011) Distinct pathways for modification of the bacterial cell wall by non-canonical d-amino acids. EMBO J 30:3442–3453
Chamond N, Grégoire C, Coatnoan N, Rougeot C, Freitas-Junior LH, da Silveira JF, Degrave WM, Minoprio P (2003) Biochemical characterization of proline racemases from the human protozoan parasite Trypanosoma cruzi and definition of putative protein signatures. J Biol Chem 278:15484–15494
Chang YF, Adams E (1974) d-lysine catabolic pathway in Pseudomonas putida: interrelations with l-lysine catabolism. J Bacteriol 117:753–764
Collins LV, Kristian SA, Weidenmaier C, Faigle M, van Kessel KPM, van Strijp JAG, Götz F, Neumeister B, Peschel A (2002) Staphylococcus aureus strains lacking d-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J Infect Dis 186:214–219
Conti P, Tamborini L, Pinto A, Blondel A, Minoprio P, Mozzarelli A, De Micheli C (2011) Drug discovery targeting amino acid racemases. Chem Rev 111:6919–6946
Cotter PD, O’Connor PM, Draper LA, Lawton EM, Deegan LH, Hill C, Ross RP (2005) Posttranslational conversion of l-serines to d-alanines is vital for optimal production and activity of the lantibiotic lacticin 3147. Proc Natl Acad Sci U S A 102:18584–18589
Dawson DW, Volpert OV, Pearce SF, Schneider AJ, Silverstein RL, Henkin J, Bouck NP (1999) Three distinct d-amino acid substitutions confer potent antiangiogenic activity on an inactive peptide derived from a thrombospondin-1 type 1 repeat. Mol Pharmacol 55:332–338
Dey M, Patra S, Su LY, Segall AM (2013) Tumor cell death mediated by peptides that recognize branched intermediates of DNA replication and repair. PLoS One 8:e78751
Doublet P, van Heijenoort J, Bohin J, Mengin-Lecreulx D (1993) The murI gene of Escherichia coli is an essential gene that encodes a glutamate racemase activity. J Bacteriol 175:2970
Espaillat A, Carrasco-López C, Bernardo-García N, Pietrosemoli N, Otero LH, Álvarez L, de Pedro MA, Pazos F, Davis BM, Waldor MK, Hermoso JA, Cava F (2014) Structural basis for the broad specificity of a new family of amino-acid racemases. Acta Crystallogr D Biol Crystallogr 70:79–90
Fang FC, Libby SJ, Buchmeier NA, Loewen PC, Switala J, Harwood J, Guiney DG (1992) The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc Natl Acad Sci U S A 89:11978–11982
Fernandez-Lopez S, Kim HS, Choi EC, Delgado M, Granja JR, Khasanov A, Kraehenbuehl K, Long G, Weinberger DA, Wilcoxen KM, Ghadiri MR (2001) Antibacterial agents based on the cyclic d, l-α-peptide architecture. Nature 412:452–455
Fisher SL (2008) Glutamate racemase as a target for drug discovery. Microb Biotechnol 1:345–360
Fotheringham I, Bledig S, Taylor P (1998) Characterization of the genes encoding d-amino acid transaminase and glutamate racemase, two d-glutamate biosynthetic enzymes of Bacillus sphaericus ATCC 10208. J Bacteriol 180:4319
Gallo KA, Knowles JR (1993) Purification, cloning, and cofactor independence of glutamate racemase from Lactobacillus. Biochemistry 32:3981–3990
Glavas S, Tanner ME (1999) Catalytic acid/base residues of glutamate racemase. Biochemistry 38:4106–4113
Goytia M, Chamond N, Cosson A, Coatnoan N, Hermant D, Berneman A, Minoprio P (2007) Molecular and structural discrimination of proline racemase and hydroxyproline-2-epimerase from nosocomial and bacterial pathogens. PLoS One 2:e885
Gryder R, Adams E (1969) Inducible degradation of hydroxyproline in Pseudomonas putida: pathway regulation and hydroxyproline uptake. J Bacteriol 97:292–306
Güell I, Cabrefiga J, Badosa E, Ferre R, Talleda M, Bardají E, Planas M, Feliu L, Montesinos E (2011) Improvement of the efficacy of linear undecapeptides against plant-pathogenic bacteria by incorporation of d-amino acids. Appl Environ Microbiol 77:2667–2675
Guinand M, Ghuysen J-M, Schleifer KH, Kandlert O (1969) The peptidoglycan in walls of Butyribacterium rettgeri. Biochemistry 8:200–207
He W, Li C, Lu C-D (2011) Regulation and characterization of the dadRAX locus for d-amino acid catabolism in Pseudomonas aeruginosa PAO1. J Bacteriol 193:2107–2115
Hills GM (1949) Chemical factors in the germination of spore-bearing aerobes; the effect of yeast extract on the germination of Bacillus anthracis and its replacement by adenosine. Biochem J 45:353–362
Hochbaum AI, Kolodkin-Gal I, Foulston L, Kolter R, Aizenberg J, Losick R (2011) Inhibitory effects of d-amino acids on Staphylococcus aureus biofilm development. J Bacteriol 193:5616–5622
Hu H, Emerson J, Aronson AI (2007) Factors involved in the germination and inactivation of Bacillus anthracis spores in murine primary macrophages. FEMS Microbiol Lett 272:245–250
Ichihara A, Furiya S, Suda M (1960) Metabolism of l-lysine by bacterial enzymes. J Biochem 48:277–283
Kato S, Ishihara T, Hemmi H, Kobayashi H, Yoshimura T (2011) Alterations in d-amino acid concentrations and microbial community structures during the fermentation of red and white wines. J Biosci Bioeng 111:104–108
Kato S, Hemmi H, Yoshimura T (2012) Lysine racemase from a lactic acid bacterium, Oenococcus oeni: structural basis of substrate specificity. J Biochem 152:505–508
Kawai Y, Ishii Y, Arakawa K, Uemura K, Saitoh B, Nishimura J, Kitazawa H, Yamazaki Y, Tateno Y, Itoh T, Saito T (2004) Structural and functional differences in two cyclic bacteriocins with the same sequences produced by lactobacilli. Appl Environ Microbiol 70:2906–2911
Keller U, Schauwecker F (2001) Nonribosomal biosynthesis of microbial chromopeptides. Prog Nucleic Acid Res Mol Biol 70:233–289
Kimura K, Tran L-SP, Itoh Y (2004) Roles and regulation of the glutamate racemase isogenes, racE and yrpC, in Bacillus subtilis. Microbiology 150:2911–2920
Kobayashi J, Shimizu Y, Mutaguchi Y, Doi K, Ohshima T (2013) Characterization of d-amino acid aminotransferase from Lactobacillus salivarius. J Mol Catal B Enzym 94:15–22
Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R (2010) d-amino acids trigger biofilm disassembly. Science 328:627–629
Konz D, Klens A, Schörgendorfer K, Marahiel MA (1997) The bacitracin biosynthesis operon of Bacillus licheniformis ATCC 10716: molecular characterization of three multi-modular peptide synthetases. Chem Biol 4:927–937
Kristian S, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo R, Nizet V (2005) d-Alanylation of teichoic acids promotes group A Streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J Bacteriol 187:6719
Kuan Y-C, Kao C-H, Chen C-H, Chen C-C, Hu H-Y, Hsu W-H (2011) Biochemical characterization of a novel lysine racemase from Proteus mirabilis BCRC10725. Process Biochem 46:1914–1920
Kuramitsu H, Snoke J (1962) The biosynthesis of d-amino acids in Bacillus licheniformis. Biochim Biophys Acta 62:114–121
Kuru E, Hughes HV, Brown PJ, Hall E, Tekkam S, Cava F, de Pedro MA, Brun YV, VanNieuwenhze MS (2012) In situ probing of newly synthesized peptidoglycan in live bacteria with fluorescent d-amino acids. Angew Chem Int Ed Engl 51:12519–12523
Lam H, Oh D-C, Cava F, Takacs CN, Clardy J, de Pedro MA, Waldor MK (2009) d-amino acids govern stationary phase cell wall remodeling in bacteria. Science 325:1552–1555
Lamont H, Staudenbauer W, Strominger J (1972) Partial purification and characterization of an aspartate racemase from Streptococcus faecalis. J Biol Chem 247:5103–5106
Leiman SA, May JM, Lebar MD, Kahne D, Kolter R, Losick R (2013) d-Amino acids indirectly inhibit biofilm formation in Bacillus subtilis by interfering with protein synthesis. J Bacteriol 195:5391–5395
Li J, Jensen SE (2008) Nonribosomal biosynthesis of fusaricidins by Paenibacillus polymyxa PKB1 involves direct activation of a d-amino acid. Chem Biol 15:118–127
Li C, Yao X, Lu C-D (2010) Regulation of the dauBAR operon and characterization of d-amino acid dehydrogenase DauA in arginine and lysine catabolism of Pseudomonas aeruginosa PAO1. Microbiology 156:60–71
Liechti GW, Kuru E, Hall E, Kalinda A, Brun YV, VanNieuwenhze M, Maurelli AT (2014) A new metabolic cell-wall labelling method reveals peptidoglycan in Chlamydia trachomatis. Nature 506:507–510
Liu L, Iwata K, Kita A, Kawarabayasi Y, Yohda M, Miki K (2002) Crystal structure of aspartate racemase from Pyrococcus horikoshii OT3 and its implications for molecular mechanism of PLP-independent racemization. J Mol Biol 319:479–489
Lobocka M, Hennig J, Wild J, Klopotowski T (1994) Organization and expression of the Escherichia coli K-12 dad operon encoding the smaller subunit of d-amino acid dehydrogenase and the catabolic alanine racemase. J Bacteriol 176:1500
Lupoli T, Tsukamoto H, Doud E, Andrew Wang T-S, Walker S, Kahne D (2011) Transpeptidase-mediated incorporation of d-amino acids into bacterial peptidoglycan. J Am Chem Soc 133:10748–10751
Marahiel MA, Krause M, Skarpeid HJ (1985) Cloning of the tyrocidine synthetase 1 gene from Bacillus brevis and its expression in Escherichia coli. Mol Gen Genet 201:231–236
Martín JF, Gutiérrez S, Fernández FJ, Velasco J, Fierro F, Marcos AT, Kosalkova K (1994) Expression of genes and processing of enzymes for the biosynthesis of penicillins and cephalosporins. Antonie Van Leeuwenhoek 65:227–243
Massey LK, Sokatch JR, Conrad RS (1976) Branched-chain amino acid catabolism in bacteria. Bacteriol Rev 40:42–54
Matsui D, Oikawa T, Arakawa N, Osumi S, Lausberg F, Stäbler N, Freudl R, Eggeling L (2009) A periplasmic, pyridoxal-5′-phosphate-dependent amino acid racemase in Pseudomonas taetrolens. Appl Microbiol Biotechnol 83:1045–1054
Muramatsu H, Mihara H, Kakutani R, Yasuda M, Ueda M, Kurihara T, Esaki N (2005) The putative malate/lactate dehydrogenase from Pseudomonas putida is an NADPH-dependent Δ1-piperideine-2-carboxylate/Δ1-pyrroline-2-carboxylate reductase involved in the catabolism of d-lysine and d-proline. J Biol Chem 280:5329–5335
Murooka Y, Yamshita M (2008) Traditional healthful fermented products of Japan. J Ind Microbiol Biotechnol 35:791–798
Mutaguchi Y, Ohmori T, Wakamatsu T, Doi K, Ohshima T (2013) Identification, purification, and characterization of a novel amino acid racemase, isoleucine 2-epimerase, from Lactobacillus species. J Bacteriol 195:5207–5215
Nakajima N, Tanizawa K, Tanaka H, Soda K (1986) Cloning and expression in Escherichia coli of the glutamate racemase gene from Pediococcus pentosaceus. Agric Biol Chem 50:2823–2830
Narrod S, Wood W (1952) Evidence for a glutamic acid racemase in Lactobacillus arabinosus. Arch Biochem Biophys 35:462–463
Navab M (2002) Oral administration of an apo A–I mimetic peptide synthesized from d-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation 105:290–292
Neuhaus F, Baddiley J (2003) A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in Gram-positive bacteria. Microbiol Mol Biol Rev 67:686
Nishizawa T, Asayama M, Shirai M (2001) Cyclic heptapeptide microcystin biosynthesis requires the glutamate racemase gene. Microbiology 147:1235–1241
Palmer DR, Garrett JB, Sharma V, Meganathan R, Babbitt PC, Gerlt JA (1999) Unexpected divergence of enzyme function and sequence: “N-acylamino acid racemase” is o-succinylbenzoate synthase. Biochemistry 38:4252–4258
Pierce KJ, Salifu SP, Tangney M (2008) Gene cloning and characterization of a second alanine racemase from Bacillus subtilis encoded by yncD. FEMS Microbiol Lett 283:69–74
Pollegioni L, Molla G, Sacchi S, Rosini E, Verga R, Pilone MS (2008) Properties and applications of microbial d-amino acid oxidases: current state and perspectives. Appl Microbiol Biotechnol 78:1–16
Pucci MJ, Thanassi JA, Ho HT, Falk PJ, Dougherty TJ (1995) Staphylococcus haemolyticus contains two d-glutamic acid biosynthetic activities, a glutamate racemase and a d-amino acid transaminase. J Bacteriol 177:336–342
Radkov AD, Moe LA (2013) Amino acid racemization in Pseudomonas putida KT2440. J Bacteriol 195:5016–5024
Reichmann NT, Cassona CP, Gründling A (2013) Revised mechanism of d-alanine incorporation into cell wall polymers in Gram-positive bacteria. Microbiology 159:1868–1877
Revelles O, Espinosa-Urgel M, Fuhrer T, Sauer U, Ramos JL (2005) Multiple and interconnected pathways for l-lysine catabolism in Pseudomonas putida KT2440. J Bacteriol 187:7500–7510
Revelles O, Wittich R-M, Ramos JL (2007) Identification of the initial steps in d-lysine catabolism in Pseudomonas putida. J Bacteriol 189:2787–2792
Salifu S, Pierce K, Tangney M (2008) Cloning and analysis of two alanine racemase genes from Bacillus licheniformis. Ann Microbiol 58:287–291
Sanchez Z, Tani A, Kimbara K (2013) Extensive reduction of cell viability and enhanced matrix production in Pseudomonas aeruginosa PAO1 flow biofilms treated with a d-amino acid mixture. Appl Environ Microbiol 79:1396–1399
Schieber A, Brückner H, Ling JR (1999) GC-MS analysis of diaminopimelic acid stereoisomers and amino acid enantiomers in rumen bacteria. Biomed Chromatogr 13:46–50
Schmidt DM, Hubbard BK, Gerlt JA (2001) Evolution of enzymatic activities in the enolase superfamily: functional assignment of unknown proteins in Bacillus subtilis and Escherichia coli as l-ala-d/l-glu epimerases. Biochemistry 40:15707–15715
Schreier H (1993) Biosynthesis of glutamine and glutamate and the assimilation of ammonia. In: Hoch J, Losick R (eds) Bacillus subtilis and other Gram-positive bacteria. American Society for Microbiology, Washington DC, pp 281–298
Sharma RK, Sundriyal S, Wangoo N, Tegge W, Jain R (2010) New antimicrobial hexapeptides: synthesis, antimicrobial activities, cytotoxicity, and mechanistic studies. ChemMedChem 5:86–95
Siegrist MS, Whiteside S, Jewett JC, Aditham A, Cava F, Bertozzi CR (2013) d-amino acid chemical reporters reveal peptidoglycan dynamics of an intracellular pathogen. ACS Chem Biol 8:500–505
Soto C, Kindy MS, Baumann M, Frangione B (1996) Inhibition of Alzheimer’s amyloidosis by peptides that prevent β-sheet conformation. Biochem Biophys Res Commun 226:672–680
Stadtman T, Elliot P (1957) Studies on the enzymatic reduction of amino acids II. Purification and properties of a d-proline reductase and a proline racemase from Clostridium sticklandii. J Biol Chem 228:983–997
Stein T, Kluge B, Vater J, Franke P, Otto A, Wittmann-Liebold B (1995) Gramicidin S synthetase 1 (phenylalanine racemase), a prototype of amino acid racemases containing the cofactor 4′-phosphopantetheine. Biochemistry 34:4633–4642
Stein DB, Linne U, Marahiel MA (2005) Utility of epimerization domains for the redesign of nonribosomal peptide synthetases. FEBS J 272:4506–4520
Tang G-L, Cheng Y-Q, Shen B (2007) Chain initiation in the leinamycin-producing hybrid nonribosomal peptide/polyketide synthetase from Streptomyces atroolivaceus S-140. Discrete, monofunctional adenylation enzyme and peptidyl carrier protein that directly load d-alanine. J Biol Chem 282:20273–20282
Tanner M (2002) Understanding nature’s strategies for enzyme-catalyzed racemization and epimerization. Acc Chem Res 35:237–246
Thompson A, Griffin H, Gasson MJ (2002) Characterization of an alanine racemase gene from Lactobacillus reuteri. Curr Microbiol 44:246–250
Tokuyama S, Hatano K (1995) Purification and properties of thermostable N-acylamino acid racemase from Amycolatopsis sp. TS-1-60. Appl Microbiol Biotechnol 42:853–859
Tokuyama S, Hatano K, Takahashi T (1994) Discovery of a novel enzyme, N-acylamino acid racemase in an Actinomycete: screening, isolation, and identification. Biosci Biotechnol Biochem 58:24–27
Toney MD (2011) Controlling reaction specificity in pyridoxal phosphate enzymes. Biochim Biophys Acta 1814:1407–1418
Umbarger H (1978) Amino acid biosynthesis and its regulation. Annu Rev Biochem 47:533–606
Veiga P, Piquet S, Maisons A, Furlan S, Courtin P, Chapot-Chartier M-P, Kulakauskas S (2006) Identification of an essential gene responsible for d-asp incorporation in the Lactococcus lactis peptidoglycan crossbridge. Mol Microbiol 62:1713–1724
Volkman BF, Zhang Q, Debabov DV, Rivera E, Kresheck GC, Neuhaus FC (2001) Biosynthesis of d-alanyl-lipoteichoic acid: the tertiary structure of apo-d-alanyl carrier protein. Biochemistry 40:7964–7972
Vollmer W, Blanot D, de Pedro MA (2008) Peptidoglycan structure and architecture. FEMS Microbiol Rev 32:149–167
Vranova V, Zahradnickova H, Janous D, Skene KR, Matharu AS, Rejsek K, Formanek P (2011) The significance of d-amino acids in soil, fate and utilization by microbes and plants: review and identification of knowledge gaps. Plant Soil 354:21–39
Walton CJW, Chica RA (2013) A high-throughput assay for screening l- or d-amino acid specific aminotransferase mutant libraries. Anal Biochem 441:190–198
Wasserman SA, Walsh CT, Botstein D (1983) Two alanine racemase genes in Salmonella typhimurium that differ in structure and function. J Bacteriol 153:1439–1450
Wei G, Bobek L (2005) Human salivary mucin MUC7 12-mer-l and 12-mer-d peptides: antifungal activity in saliva, enhancement of activity with protease inhibitor cocktail or EDTA, and cytotoxicity to human cells. Antimicrob Agents Chemother 49:2336
Welch BD, VanDemark AP, Heroux A, Hill CP, Kay MS (2007) Potent d-peptide inhibitors of HIV-1 entry. Proc Natl Acad Sci U S A 104:16828–16833
White CE, Gavina JMA, Morton R, Britz-McKibbin P, Finan TM (2012) Control of hydroxyproline catabolism in Sinorhizobium meliloti. Mol Microbiol 85:1133–1147
Wu H-M, Kuan Y-C, Chu C-H, Hsu W-H, Wang W-C (2012) Crystal structures of lysine-preferred racemases, the non-antibiotic selectable markers for transgenic plants. PLoS One 7:e48301
Yamashita T, Ashiuchi M, Ohnishi K, Kato S, Nagata S, Misono H (2004) Molecular identification of monomeric aspartate racemase from Bifidobacterium bifidum. Eur J Biochem 271:4798–4803
Yang Z, Lu C-D (2007) Functional genomics enables identification of genes of the arginine transaminase pathway in Pseudomonas aeruginosa. J Bacteriol 189:3945–3953
Yin X, Zabriskie TM (2006) The enduracidin biosynthetic gene cluster from Streptomyces fungicidicus. Microbiology 152:2969–2983
Yonaha K, Misono H, Yamamoto T, Soda K (1975) d-amino acid aminotransferase of Bacillus sphaericus. Enzymologic and spectrometric properties. J Biol Chem 250:6983–6989
Acknowledgments
Research on d-amino acid synthesis in the PI’s lab is funded in part by a grant from the National Institute of Food and Agriculture of the US Department of Agriculture (grant 2011-67020-30195).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 54 kb)
Rights and permissions
About this article
Cite this article
Radkov, A.D., Moe, L.A. Bacterial synthesis of d-amino acids. Appl Microbiol Biotechnol 98, 5363–5374 (2014). https://doi.org/10.1007/s00253-014-5726-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5726-3