Abstract
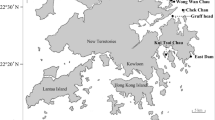
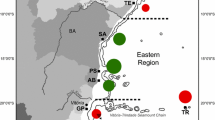
The scleractinian reef building coral Madracis decactis is a cosmopolitan species. Understanding host–symbiont associations is critical for assessing coral’s habitat requirements and its response to environmental changes. In this study, we performed a fine grained phylogenetic analyses of Symbiodiniaceae associated with Madracis in two locations in the Southwest Atlantic Ocean (Abrolhos Bank and St. Peter and St. Paul Archipelago). Previous studies have argued that Madracis is a specialist coral, with colonies harboring a single symbiont from the genus Breviolum (formerly clade B). However, these previous studies have not precisely addressed if Madracis is colonized by several types of Symbiodiniaceae simultaneously or whether this coral is a specialist. The hypothesis that Madracis is a generalist coral host was evaluated in the present study. A total of 1.9 million reads of ITS2 nuclear ribosomal DNA were obtained by Illumina MiSeq sequencing. While Symbiodiniaceae ITS2 sequences between two sampling depths were almost entirely (62%) from the genus Breviolum (formerly clade B), shallow (10–15 m) populations in Abrolhos had a greater diversity of ITS2 sequences in comparison to deeper (25–35 m) populations of St. Peter and St. Paul Archipelago. Cladocopium (formerly clade C) and Symbiodinium (formerly clade A) were also found in Abrolhos. A single Madracis colony can host different symbiont types with > 30 Symbiodiniaceae ITS2-type profiles. Abrolhos corals presented a higher photosynthetic potential as a possible result of co-occurrence of multiple Symbiodiniaceae in a single coral colony. Multiple genera/clades of Symbiodiniaceae possibly confer coral hosts with broader environmental tolerance and ability to occupy diverse or changing habitats.




Similar content being viewed by others
Data Availability
Raw ITS2 amplicon sequences are available in the National Center for Biotechnology Information (NCBI), Sequence Read Archive (SRA) under the Project No. PRJNA732715 and Accession Nos. SAMN19340223, SAMN19340224, SAMN19340225, SAMN19340226, SAMN19340239, SAMN19340240, and SAMN19340241.
Code Availability
Not applicable.
References
LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR (2018) Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol 28(16):2570–2580. https://doi.org/10.1016/j.cub.2018.07.008
Suggett DJ, Warner ME, Leggat W (2017) Symbiotic dinoflagellate functional diversity mediates coral survival under ecological crisis. Trends Ecol Evol 32(10):735–745. https://doi.org/10.1016/j.tree.2017.07.013
Jokiel PL, Brown EK (2004) Global warming, regional trends and inshore environmental conditions influence coral bleaching in Hawaii. Glob Change Biol 10(10):1627–1641. https://doi.org/10.1111/j.1365-2486.2004.00836.x
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80(4):435–471. https://doi.org/10.1016/j.ecss.2008.09.003
Coffroth MA, Santos SR (2005) Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist 156(1):19–34. https://doi.org/10.1016/j.protis.2005.02.004
Pochon X, Gates RD (2010) A new Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawai’i. Mol Phylogenet Evol 56(1):492–497. https://doi.org/10.1016/j.ympev.2010.03.040
Yao H, Song J, Liu C, Luo K, Han J, Li Y et al (2010) Use of ITS2 region as the universal DNA barcode for plants and animals. PLoS ONE 5:e13102. https://doi.org/10.1371/journal.pone.0013102
Moorhouse-Gann RJ, Dunn JC, De Vere N, Goder M, Cole N, Hipperson H, Symondson WO (2018) New universal ITS2 primers for high-resolution herbivory analyses using DNA metabarcoding in both tropical and temperate zones. Sci Rep 8(1):1–15. https://doi.org/10.1038/s41598-018-26648-2
Oliver TA, Palumbi SR (2011) Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30(2):429–440. https://doi.org/10.1007/s00338-011-0721-y
Varasteh T, Shokri MR, Rajabi-Maham H, Behzadi S, Hume BC (2018) Symbiodinium thermophilum symbionts in Porites harrisoni and Cyphastrea microphthalma in the northern Persian Gulf, Iran. Marine Biological Association of the United Kingdom. J Mar Biolog Assoc UK 98(8):2067–2073. https://doi.org/10.1017/S0025315417001746
Diekmann O, Olsen J, Stam W, Bak R (2003) Genetic variation within Symbiodinium clade B from the coral genus Madracis in the Caribbean (Netherlands Antilles). Coral Reefs 22(1):29–33. https://doi.org/10.1007/s00338-002-0273-2
Frade PR, Englebert N, Faria J, Visser PM, Bak RPM (2008) Distribution and photobiology of Symbiodinium types in different light environments for three colour morphs of the coral Madracis pharensis: is there more to it than total irradiance? Coral Reefs 27(4):913–925. https://doi.org/10.1007/s00338-008-0406-3
Rodriguez-Lanetty M, Loh W, Carter D, Hoegh-Guldberg O (2001) Latitudinal variability in symbiont specificity within the widespread scleractinian coral Plesiastrea versipora. Mar Biol 138(6):1175. https://doi.org/10.1007/s002270100536
Knowlton N, Rohwer F (2003) Multispecies microbial mutualisms on coral reefs: the host as a habitat. The American naturalist, 162(S4), S51-S62. https://www.jstor.org/stable/https://doi.org/10.1086/378684.
Garren M, Walsh SM, Caccone A, Knowlton N (2006) Patterns of association between Symbiodinium and members of the Montastraea annularis species complex on spatial scales ranging from within colonies to between geographic regions. Coral Reefs 25(4):503–512. https://doi.org/10.1007/s00338-006-0146-1
Valentin JL (2001) The Cabo Frio upwelling system, Brazil. In Coastal marine ecosystems of Latin America (pp. 97–105). Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-04482-7_8.
Silva-Lima AW, Walter JM, Garcia GD, Ramires N, Ank G, Meirelles PM, Thompson FL (2015) Multiple Symbiodinium strains are hosted by the Brazilian endemic corals Mussismilia spp. Microb Ecol 70(2):301–310. https://doi.org/10.1007/s00248-015-0573-z
Picciani N, e Seiblitz IGDL, de Paiva PC, e Castro CB, Zilberberg C (2016) Geographic patterns of Symbiodinium diversity associated with the coral Mussismilia hispida (Cnidaria, Scleractinia) correlate with major reef regions in the Southwestern Atlantic Ocean. Mar Biol 163(11):1–11. https://doi.org/10.1007/s00227-016-3010-z
Baker AC (2003) Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Evol Syst 34(1):661–689. https://doi.org/10.1146/annurev.ecolsys.34.011802.132417
Monteiro JG, Costa CF, Gorlach-Lira K, Fitt WK, Stefanni SS, Sassi R, LaJeunesse TC (2013) Ecological and biogeographic implications of Siderastrea symbiotic relationship with Symbiodinium sp. C46 in Sal Island (Cape Verde, East Atlantic Ocean). Mar Biodivers 43(4):261–272. https://doi.org/10.1007/s12526-013-0153-8
Mies M, Francini-Filho RB, Zilberberg C, Garrido AG, Longo GO, Laurentino E, Banha TN (2020) South Atlantic coral reefs are major global warming refugia and less susceptible to bleaching. Front Mar Sci 7:514. https://doi.org/10.3389/fmars.2020.00514
Moreira APB, Meirelles PM, Santos EDO, Amado-Filho GM, Francini-Filho RB, Thompson CC, Thompson FL (2015) Turbulence-driven shifts in holobionts and planktonic microbial assemblages in St. Peter and St. Paul Archipelago, Mid-Atlantic Ridge Brazil. Frontiers in microbiology 6:1038. https://doi.org/10.3389/fmicb.2015.01038
Neves E, Johnsson R (2009) Taxonomic revision of the southwestern Atlantic Madracis and the description of Madracis fragilis n. sp.(Scleractinia: Pocilloporidae), a new coral species from Brazil. Scientia Marina, 73(4), 739-746. http://www.repositorio.ufba.br/ri/handle/ri/13116.
Francini-Filho RB, Coni EO, Meirelles PM, Amado-Filho GM, Thompson FL, Pereira-Filho GH, Bastos AC, Abrantes DP, Ferreira CM, Gibran FZ, Güth AZ (2013) Dynamics of coral reef benthic assemblages of the Abrolhos Bank, eastern Brazil: inferences on natural and anthropogenic drivers. PLoS ONE 8(1):e54260. https://doi.org/10.1371/journal.pone.0054260
Nunes LT, Cord I, Francini-Filho RB, Stampar SN, Pinheiro HT, Rocha LA, Floeter SR, Ferreira CE (2019) Ecology of Prognathodes obliquus, a butterflyfish endemic to mesophotic ecosystems of St. Peter and St. Paul’s Archipelago. Coral Reefs 38(5):955–960. https://doi.org/10.1007/s00338-019-01822-8
Moreira APB, Tonon LAC, Cecilia do Valle PP, Alves N, Amado-Filho GM, Francini-Filho RB, Thompson FL (2014). Culturable heterotrophic bacteria associated with healthy and bleached scleractinian Madracis decactis and the fire worm Hermodice carunculata from the remote St. Peter and St. Paul Archipelago, Brazil. Current Microbiology, 68(1), 38-46. https://doi.org/10.1007/s00284-013-0435-1
Magalhães GM, Amado-Filho GM, Rosa MR, de Moura RL, Brasileiro PS, De Moraes FC, Francini-Filho RB, Pereira-Filho GH (2015) Changes in benthic communities along a 0–60 m depth gradient in the remote St. Peter and St. Paul Archipelago (Mid-Atlantic Ridge, Brazil). Bull Mar Sci 91(3):377–396. https://doi.org/10.5343/bms.2014.1044
LaJeunesse TJMB (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141(2):387–400. https://doi.org/10.1007/s00227-002-0829-2
Coffroth MA, Lasker HR, Diamond ME et al (1992) DNA fingerprints of a gorgonian coral: a method for detecting clonal structure in a vegetative species. Mar Biol 114:317–325. https://doi.org/10.1007/BF00349534
Pochon X, Pawlowski J, Zaninetti L, Rowan R (2001) High genetic diversity and relative specificity among Symbiodinium-like endosymbiotic dinoflagellates in soritid foraminiferans. Mar. Biol. 139:1069–1078. https://doi.org/10.1007/s002270100674
Quail MA, Smith M, Coupland P, Otto TD, Harris SR, Connor TR, Gu Y (2012) A tale of three next generation sequencing platforms: comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genomics 13(1):1–13. https://doi.org/10.1186/1471-2164-13-341
Hume BC, Smith EG, Ziegler M, Warrington HJ, Burt JA, LaJeunesse TC, Voolstra CR (2019) SymPortal: a novel analytical framework and platform for coral algal symbiont next-generation sequencing ITS2 profiling. Mol Ecol Resour 19(4):1063–1080. https://doi.org/10.1111/1755-0998.13004
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541. https://doi.org/10.1128/AEM.01541-09
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL (2009) BLAST+: architecture and applications. BMC Bioinformatics 10(1):1–9. https://doi.org/10.1186/1471-2105-10-421
Eren AM, Morrison HG, Lescault PJ, Reveillaud J, Vineis JH, Sogin ML (2015) Minimum entropy decomposition: unsupervised oligotyping for sensitive partitioning of high-throughput marker gene sequences. ISME J 9(4):968–979. https://doi.org/10.1038/ismej.2014.195
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32(5):1792–1797. https://doi.org/10.1093/nar/gkh340
Price MN, Dehal PS, Arkin AP (2010) FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE 5(3):e9490. https://doi.org/10.1371/journal.pone.0009490
McKinney, Wes (2010) Data structures for statistical computing in python. Proceedings of the 9th Python in Science Conference. Vol. 445. https://doi.org/10.25080/Majora-92bf1922-00a.
Hunter J (2007) Matplotlib: A 2D Graphics Environment in Computing in Science & Engineering, vol. 9, no. 03, pp. 90–95. Keywords: {python;scripting languages;application development;scientific programming}url:https://doi.ieeecomputersociety.org/https://doi.org/10.1109/MCSE.2007.55. https://doi.org/10.1109/MCSE.2007.55.
Huerta-Cepas J, François S, Bork P (2016) ETE 3: reconstruction, analysis, and visualization of phylogenomic data. Mol Biol Evol 33(6):1635–1638. https://doi.org/10.1093/molbev/msw046
Zar JH (1999) Biostatistical analysis. Pearson Education India.
Reis VM, Karez CS, Mariath R, de Moraes FC, de Carvalho RT, Brasileiro PS, Bahia Rda G, Lotufo TM, Ramalho LV, de Moura RL, Francini-Filho RB, Pereira-Filho GH, Thompson FL, Bastos AC, Salgado LT, Amado-Filho GM (2016) Carbonate production by benthic communities on shallow coralgal reefs of Abrolhos Bank Brazil. PLoS One. 11(4):e0154417. https://doi.org/10.1371/journal.pone.0154417
Silverstein RN, Cunning R, Baker AC (2017) Tenacious D: Symbiodinium in clade D remain in reef corals at both high and low temperature extremes despite impairment. J Exp Biol 220(7):1192–1196. https://doi.org/10.1242/jeb.148239
Swain TD, Chandler J, Backman V, Marcelino L (2017) Consensus thermotolerance ranking for 110 Symbiodinium phylotypes: an exemplar utilization of a novel iterative partial-rank aggregation tool with broad application potential. Funct Ecol 31(1):172–183. https://doi.org/10.1111/1365-2435.12694
Eckert RJ, Studivan MS, Voss JD (2019) Populations of the coral species Montastraea cavernosa on the Belize Barrier Reef lack vertical connectivity. Sci Rep 9(1):1–11. https://doi.org/10.1038/s41598-019-43479-x
Teschima MM, Garrido A, Paris A, Nunes FL, Zilberberg C (2019) Biogeography of the endosymbiotic dinoflagellates (Symbiodiniaceae) community associated with the brooding coral Favia gravida in the Atlantic Ocean. PLoS ONE 14(3):e0213519. https://doi.org/10.1371/journal.pone.0213519
Eckert RJ, Reaume AM, Sturm AB, Studivan MS, Voss JD (2020) Depth influences Symbiodiniaceae associations among Montastraea cavernosa corals on the Belize Barrier Reef. Front Microbiol 11:518. https://doi.org/10.3389/fmicb.2020.00518
Bongaerts P, Frade PR, Hay KB, Englebert N, Latijnhouwers KR, Bak RP, Hoegh-Guldberg O (2015) Deep down on a Caribbean reef: lower mesophotic depths harbor a specialized coral-endosymbiont community. Sci Rep 5(1):1–9. https://doi.org/10.1038/srep07652
Thornhill DJ, Kemp DW, Bruns BU, Fitt WK, Schmidt GW (2008) Correspondence between cold tolerance and temperate biogeography in a Western Atlantic Symbiodinium (Dinophyta) lineage 1. J Phycol 44(5):1126–1135. https://doi.org/10.1111/j.1529-8817.2008.00567.x
Adams LM, Cumbo VR, Takabayashi M (2009) Exposure to sediment enhances primary acquisition of Symbiodinium by asymbiotic coral larvae. Mar Ecol Prog Ser 377:149–156. https://doi.org/10.3354/meps07834
Loram JE, Trapido-Rosenthal HG, Douglas AE (2007) Functional significance of genetically different symbiotic algae Symbiodinium in a coral reef symbiosis. Mol Ecol 16(22):4849–4857. https://doi.org/10.1111/j.1365-294X.2007.03491.x
Davies SW, Ries JB, Marchetti A, Granzotti R, Castillo KD (2017) Symbiodinium functional diversity and clade specificity under global change stressors Running Title (50 characters max): In-hospite Symbiodinium transcriptomes under stress. https://doi.org/10.1101/190413.
Acknowledgements
We are grateful for the support offered by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Pesquisas (CNPq), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Funding
Support was offered by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), ConselhomNacional de Pesquisas (CNPq), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Author information
Authors and Affiliations
Contributions
Tooba Varasteh conceived the study design, DNA extractions and PCR, the bioinformatics analysis, and discussion of the results and drafted the manuscript. Ronaldo Francini-Filho collected the samples in the field and participated in the acquisition of funding. Vinícius Salazar performed the bioinformatics analyses. Ronaldo Francini-Filho, Jean Swings, Diogo Tschoeke, Gizele Garcia, and Cristiane Thompson participated in the discussion of the results and drafted the manuscript. Fabiano Lopes Thompson participated in the acquisition of funding and conceived the study design, discussion of the results, and draft of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Varasteh, T., Salazar, V., Tschoeke, D. et al. Breviolum and Cladocopium Are Dominant Among Symbiodiniaceae of the Coral Holobiont Madracis decactis. Microb Ecol 84, 325–335 (2022). https://doi.org/10.1007/s00248-021-01868-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-021-01868-8