Abstract
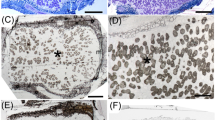
The South African invasive legume Dipogon lignosus (Phaseoleae) produces nodules with both determinate and indeterminate characteristics in New Zealand (NZ) soils. Ten bacterial isolates produced functional nodules on D. lignosus. The 16S ribosomal RNA (rRNA) gene sequences identified one isolate as Bradyrhizobium sp., one isolate as Rhizobium sp. and eight isolates as Burkholderia sp. The Bradyrhizobium sp. and Rhizobium sp. 16S rRNA sequences were identical to those of strains previously isolated from crop plants and may have originated from inocula used on crops. Both 16S rRNA and DNA recombinase A (recA) gene sequences placed the eight Burkholderia isolates separate from previously described Burkholderia rhizobial species. However, the isolates showed a very close relationship to Burkholderia rhizobial strains isolated from South African plants with respect to their nitrogenase iron protein (nifH), N-acyltransferase nodulation protein A (nodA) and N-acetylglucosaminyl transferase nodulation protein C (nodC) gene sequences. Gene sequences and enterobacterial repetitive intergenic consensus (ERIC) PCR and repetitive element palindromic PCR (rep-PCR) banding patterns indicated that the eight Burkholderia isolates separated into five clones of one strain and three of another. One strain was tested and shown to produce functional nodules on a range of South African plants previously reported to be nodulated by Burkholderia tuberum STM678T which was isolated from the Cape Region. Thus, evidence is strong that the Burkholderia strains isolated here originated in South Africa and were somehow transported with the plants from their native habitat to NZ. It is possible that the strains are of a new species capable of nodulating legumes.






Similar content being viewed by others
References
Abdel-Salem MS, Ibrahim SA, Abd-El-Halim MM, Badawy FM, Abo-Aba SEM (2010) Phenotypic characterization of indigenous Egyptian rhizobial strains for abiotic stresses performance. J Am Sci 60:498–503
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 12:39–45
Andrews M, Edwards GR, Ridgway HJ, Cameron KC, Di HJ, Raven JA (2011) Positive plant microbial interactions in perennial ryegrass dairy pasture systems. Ann Appl Biol 159:79–92
Andrews M, James EK, Sprent JI, Boddey RM, Gross E, dos Reis Jr FB (2011) Nitrogen fixation in legumes and actinorhizal plants in natural ecosystems: values obtained using 15N natural abundance. Plant Ecol Divers 4:131–140
Andrews M, Raven JA, Lea PJ (2013) Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann Appl Biol 163:174–199
Andrus AD, Andam C, Parker MA (2012) American origin of Cupriavidus bacteria associated with invasive Mimosa legumes in the Philippines. FEMS Microbiol Ecol 80:747–750
Ardley JK, Parker MA, De Meyer SE, Trengove RD, O’Hara GW, Reeve WG, Yates RJ, Dilworth MJ, Willems A, Howieson JG (2012) Microvirga lupini sp. nov., Microvirga lotononidis sp. nov., and Microvirga zambiensis sp. nov. are alphaproteobacterial root-nodule bacteria that specifically nodulate and fix nitrogen with geographically and taxonomically separate legume hosts. Int J Syst Evol Microbiol 62:2579–2588
Ardley JK, Reeve WG, O’Hara GW, Yates RJ, Dilworth MJ, Howieson JG (2013) Nodule morphology, symbiotic specificity and association with unusual rhizobia are distinguishing features of the genus Listia within the southern African crotalarioid clade Lotononis s.l. Ann Bot 112:1–15
Baethgen WE, Alley MM (1989) A manual colorimetric procedure for measuring ammonium nitrogen in soil and plant Kjeldahl digests. Commun Soil Sci Plant Anal 20:961–969
Beukes CW, Venter SN, Law IJ, Phalane FL, Steenkamp ET (2013) South African papilionoid legumes are nodulated by diverse Burkholderia with unique nodulation and nitrogen-fixation loci. PLoS One 8(7):e68406
Blakemore LC, Searle PL, Daly BK (1987) Methods for chemical analysis of soils. New Zealand Soil Bureau Scientific Report 80. Department of Scientific and Industrial Research, Lower Hutt, 103 pp
Bontemps C, Elliott GN, Simon MF, dos Reis Jr FB, Gross E, Lawton RC, Neto NE, Loureiro Mde F, de Faria SM, Sprent JI, James EK, Young JPW (2010) Burkholderia species are ancient symbionts of legumes. Mol Ecol 19:44–52
Bournaud C, de Faria SM, dos Santos JMF, Tisseyre P, Silva M, Chaintreuil C, Gross E, James EK, Prin Y, Moulin L (2013) Burkholderia species are the most common and preferred nodulating symbionts of the Piptadenia Group (tribe Mimoseae). PLoS One 8(5):e63476
Chen W-M, de Faria SM, Straliotto R, Pitard RM, Simões-Araùjo JL, Chou Y-J, Chou J-H, Barrios E, Prescott AR, Elliott GN, Sprent JI, Young JPW, James EK (2005) Proof that Burkholderia forms effective symbioses with legumes: a study of novel Mimosa-nodulating strains from South America. Appl Environ Microbiol 71:7461–7471
Chen W-M, James EK, Chou J-H, Sheu S-Y, Yang S-Z, Sprent JI (2005) β-Rhizobia from Mimosa pigra, a newly discovered invasive plant in Taiwan. New Phytol 168:661–675
Chen WM, Moulin L, Bontemps C, Vandamme P, Bena G, Boivin-Masson C (2003) Legume symbiotic nitrogen fixation by β-proteobacteria is widespread in nature. J Bacteriol 185:7266–7272
Cummings SP, Gyaneshwar P, Vinuesa P, Farruggia FT, Andrews M, Humphry D, Elliott GN, Nelson A, Orr C, Pettitt D, Shah GR, Santos SR, Krishnan HB, Odee D, Moreira FMS, Sprent JI, Young JPW, James EK (2009) Nodulation of Sesbania species by Rhizobium (Agrobacterium) strain IRBG74 and other rhizobia. Environ Microbiol 11:2510–2525
de Cunha C O, Zuleta LFG, de Almeida LGP, Ciapina LP, Borges WL, Pitard RM, Baldani JI, Straliotto R, de Faria SM, Hungria M, Cavada BS, Mercante FM, de Vasconcelos ATR (2012) Complete genome sequence of Burkholderia phenoliruptrix BR3459a (CLA1), a heat-tolerant, nitrogen-fixing symbiont of Mimosa flocculosa. J Bacteriol 194:6675–6676
De Meyer SE, Cnockaert M, Ardley JK, Trengove RD, Garau G, Howieson JG, Vandamme P (2013) Burkholderia rhynchosiae sp. nov. isolated from Rhynchosia ferulifolia root nodules from South Africa. Int J Syst Evol Microbiol 63:3944–3949
De Meyer SE, Cnockaert M, Ardley JK, Maker G, Yates R, Howieson JG, Vandamme P (2013) Burkholderia sprentiae sp. nov. isolated from Lebeckia ambigua root nodules from South Africa. Int J Syst Evol Microbiol 63:3950–3957
De Meyer SE, Cnockaert M, Ardley JK, Van Wyk B-E, Van Damme PA, Howieson JG (2014) Burkholderia dilworthii sp. nov isolated from Lebeckia ambigua root nodules from South Africa. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.058602-0
dos Reis Jr FB, Simon MF, Gross E, Boddey RM, Elliott GN, Neto NE, Loureiro Mde F, de Queiroz LP, Scotti MR, Chen W-M, Norén A, Rubio MC, de Faria SM, Bontemps C, Goi SR, Young JPW, Sprent JI, James EK (2010) Nodulation and nitrogen fixation by Mimosa spp. in the Cerrado and Caatinga biomes of Brazil. New Phytol 186:934–946
Elliott GN, Chen W-M, Chou J-H, Wang H-C, Sheu S-Y, Perin L, Reis VM, Moulin L, Simon MF, Bontemps C, Sutherland JM, Bessi R, de Faria SM, Trinick MJ, Prescott AR, Sprent JI, James EK (2007) Burkholderia phymatum is a highly effective nitrogen-fixing symbiont of Mimosa spp. and fixes nitrogen ex planta. New Phytol 173:168–180
Elliott GN, Chen W-M, Bontemps C, Chou J-H, Young JPW, Sprent JI, James EK (2007) Nodulation of Cyclopia spp. (Leguminosae, Papilionoideae) by Burkholderia tuberum. Ann Bot 100:1403–1411
Elliott GN, Chou J-H, Chen W-M, Bloemberg GV, Bontemps C, Martinez-Romero E, Velázquez E, Young JPW, Sprent JI, James EK (2009) Burkholderia spp. are the most competitive symbionts of Mimosa, particularly under N-limited conditions. Environ Microbiol 11:762–778
Fernández-López M, Goormachtig S, Gao M, D’Haeze W, van Montagu M, Holsters M (1998) Ethylene-mediated phenotypic plasticity in root nodule development on Sesbania rostrata. Proc Natl Acad Sci U S A 95:12724–12728
Frey-Klett P, Chavatte M, Clausse M-L, Courrier S, Le Roux C, Raaijmakers J, Martinotti MG, Pierrat J-C, Garbaye J (2005) Ectomycorrhizal symbiosis affects functional diversity of rhizosphere fluorescent pseudomonads. New Phytol 165:317–328
Garau G, Yates RJ, Deiana P, Howieson JG (2009) Novel strains of nodulating Burkholderia have a role in nitrogen fixation with papilionoid herbaceous legumes adapted to acid, infertile soils. Soil Biol Biochem 41:125–134
Graham PH (2008) Ecology of the root nodule bacteria of legumes. In: Dilworth MJ, James EK, Sprent JI, Newton WE (eds) Nitrogen fixing leguminous symbioses. Springer, Dordrecht, pp 23–58
Gyaneshwar P, Hirsch AM, Moulin L, Chen W-M, Elliott GN, Bontemps C, Estrada-de los Santos P, Gross E, dos Reis Jr FB, Sprent JI, Young JPW, James EK (2011) Legume-nodulating betaproteobacteria: diversity, host range, and future prospects. Mol Plant Microbe Interact 24:1276–1288
Hassen AI, Bopape FL, Habig J, Lamprecht SC (2012) Nodulation of rooibos (Aspalathus linearis Burm. f.), an indigenous South African legume, by members of both the α-proteobacteria and β-proteobacteria. Biol Fertil Soils 48:295–303
Howieson JG, De Meyer SE, Vivas-Marfisi A, Ratnayake S, Ardley JK, Yates RJ (2013) Novel Burkholderia bacteria isolated from Lebeckia ambigua—a perennial suffrutescent legume of the fynbos. Soil Biol Biochem 60:55–64
Huang B, Lv C, Zhao Y, Huang R (2012) A novel strain D5 isolated from Acacia confusa. PLoS One 7(11):e49236
Laguerre G, Nour SM, Macharet V, Sanjuan J, Drouin P, Amarger N (2001) Classification of rhizobia based on nodC and nifH analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology 147:981–993
Lewis G, Schrire B, Mackinder B, Lock M (2005) Legumes of the World. Royal Botanic Gardens, Kew
Mackereth FJH, Heron J, Talling JR (1978) Water analysis: some revised methods for limnologists. Sci Publ Freshw Biol Assoc 27:1–139
Mishra RPN, Tisseyre P, Melkonian R, Chaintreuil C, Miché L, Klonowska A, Gonzalez S, Bena G, Laguerre G, Moulin L (2012) Genetic diversity of Mimosa pudica rhizobial symbionts in soils of French Guiana: investigating the origin and diversity of Burkholderia phymatum and other beta-rhizobia. FEMS Microbiol Ecol 79:487–503
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural water. Anal Chim Acta 27:31–36
Poly F, Ranjard L, Nazaret S, Gourbière F, Monrozier LJ (2001) Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties. Appl Environ Microbiol 67:2255–2262
Popay I, Champion P, James T (2010) Common weeds of New Zealand, 3rd edn. New Zealand Plant Protection Society, Inc., Christchurch
Sarita S, Sharma PK, Priefer UB, Prell J (2005) Direct amplification of rhizobial nodC sequences from soil total DNA and comparison to nodC diversity of root nodule isolates. FEMS Microbiol Ecol 54:1–11
Sheu S-Y, Chou J-H, Bontemps C, Elliott GN, Gross E, James EK, Sprent JI, Young JPW, Chen W-M (2012) Burkholderia symbiotica sp. nov., isolated from root nodules of Mimosa spp. native to North East Brazil. Int J Syst Evol Microbiol 62:2272–2278
Sheu S-Y, Chou J-H, Bontemps C, Elliott GN, Gross E, dos Reis Jr FB, Melkonian R, Moulin L, James EK, Sprent JI, Young JPW, Chen W-M (2013) Burkholderia diazotrophica sp. nov., isolated from root nodules of Mimosa spp. Int J Syst Evol Microbiol 63:435–441
Shiraishi A, Matsushita N, Hougetsu T (2010) Nodulation in black locust by the gammaproteobacteria Pseudomonas sp. and the betaproteobacteria Burkholderia sp. Syst Appl Microbiol 33:269–274
Sprent JI (2009) Legume nodulation a global perspective. Wiley-Blackwell, Chichester
Sprent JI, James EK (2007) Legume evolution: where do nodules and mycorrhizas fit in? Plant Physiol 144:575–581
Suárez-Moreno ZR, Caballero-Mellado J, Coutinho BG, Mendonça-Previato L, James EK, Venturi V (2012) Common features of environmental and potentially beneficial plant-associated Burkholderia. Microb Ecol 63:249–266
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tan HW, Weir BS, Carter N, Heenan PB, Ridgway HJ, James EK, Sprent JI, Young JPW, Andrews M (2012) Rhizobia with 16S rRNA and nifH similar to Mesorhizobium huakuii but novel recA, glnll, nodA and nodC genes are symbionts of New Zealand Carmichaelinae. PLoS One 7(10):e47677
Taulé C, Zabaleta M, Mareque C, Platero R, Sanjurjo L, Sicardi M, Frioni L, Battistoni F, Fabiano E (2012) New betaproteobacterial rhizobium strains able to efficiently nodulate Parapiptadenia rigida (Benth.) Brenan. Appl Environ Microbiol 78:1692–1700
Vandamme P, Goris J, Chen WM, de Vos P, Willems A (2002) Burkholderia tuberum sp. nov. and Burkholderia phymatum sp. nov., nodulate the roots of tropical legumes. Syst Appl Microbiol 25:507–512
Versalovic J, Koeuth T, Lupski JR (1991) Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 19:6823–6831
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. IBP Handbook No 15. Blackwell Scientific Publications, Oxford
Vinuesa P, Silva C, Lorite MJ, Izaguirre-Mayoral ML, Bedmar EJ, Martinez-Romero E (2005) Molecular systematics of rhizobia based on maximum likelihood and Bayesian phylogenies inferred from rrs, atpD, recA and nifH sequences, and their use in the classification of Sesbania microsymbionts from Venezuelan wetlands. Syst Appl Microbiol 28:702–716
Weir BS, Turner SJ, Silvester WB, Park D-C, Young JM (2004) Unexpectedly diverse Mesorhizobium strains and Rhizobium leguminosarum nodulate native legume genera of New Zealand, while introduced legume weeds are nodulated by Bradyrhizobium species. Appl Environ Microbiol 70:5980–5987
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Young JM, Park D-C, Weir BS (2004) Diversity of 16S rDNA sequences of Rhizobium spp. implications for species determinations. FEMS Microbiol Lett 238:125–131
Acknowledgments
WYYL was financially supported by a Lincoln University doctoral scholarship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Agarose gel electrophoresis of ERIC-PCR and rep-PCR fingerprinting patterns from genomic DNA of Burkholderia sp. isolates recovered from the nodules of Dipogon lignosus and the Burkholderia phytofirmans-type strain. Lanes 1 and 20, 1-kb plus DNA ladder (Invitrogen, Australia); lanes 2-10, ERIC-PCR fingerprinting patterns; lanes 11-19, rep-PCR fingerprinting patterns. Isolates are indicated at the top of each lane (GIF 97 kb)
Rights and permissions
About this article
Cite this article
Liu, W.Y.Y., Ridgway, H.J., James, T.K. et al. Burkholderia sp. Induces Functional Nodules on the South African Invasive Legume Dipogon lignosus (Phaseoleae) in New Zealand Soils. Microb Ecol 68, 542–555 (2014). https://doi.org/10.1007/s00248-014-0427-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-014-0427-0