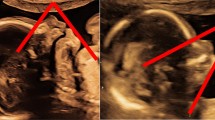
Abstract
Background
Pituitary gland height reflects secretory activity of the hypothalamo-pituitary axis.
Objective
To assess the cumulative impact of fetal growth and sex on pituitary gland height in premature twins, dissociated from prematurity.
Materials and Methods
A retrospective study was conducted, assessing the pituitary gland height in 63 pairs of preterm twins, measured from T1-weighted magnetic resonance imaging (MRI). Auxological parameters, including body weight, body length, and head circumference, at birth and at the time of MRI, were used as proxies for fetal and postnatal growth, respectively. The study population was divided into two groups, using corrected age at around term equivalent as the cutoff point. Statistical analysis was performed using mixed-effects linear regression models.
Results
When pituitary gland height was evaluated at around term equivalent, a greater pituitary gland height, suggesting a more immature hypothamo-pituitary axis, was associated with the twin exhibiting lower auxological data at birth. The same association was observed when body weight and length at MRI were used as covariants. In the group evaluated after term equivalent, a smaller pituitary gland height, suggesting a more mature hypothamo-pituitary axis, was associated with male sex. This difference was observed in twin pairs with higher average body weight at birth, and in babies exhibiting higher auxological data at MRI.
Conclusion
After isolating the effect of prematurity, at around term equivalent, pituitary gland height reflects the cumulative impact of fetal growth on the hypothalamo-pituitary axis. Subsequently, pituitary gland height shows effects of sex and of fetal and postnatal growth.
Graphical Abstract


Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Argyropoulou M, Perignon F, Brauner R, Brunelle F (1992) Magnetic resonance imaging in the diagnosis of growth hormone deficiency. J Pediatr 120:886–891
Argyropoulou M, Perignon F, Brunelle F et al (1991) Height of normal pituitary gland as a function of age evaluated by magnetic resonance imaging in children. Pediatr Radiol 21:247–249
Argyropoulou MI, Xydis V, Kiortsis DN et al (2004) Pituitary gland signal in pre-term infants during the first year of life: an MRI study. Neuroradiology 46:1031–1035
Argyropoulou MI, Kiortsis DN (2005) MRI of the hypothalamic-pituitary axis in children. Pediatr Radiol 35:1045–1055
Lurie SN, Doraiswamy PM, Husain MM et al (1990) In vivo assessment of pituitary gland volume with magnetic resonance imaging: the effect of age. J Clin Endocrinol Metab 71:505–508
Pérignon F, Brauner R, Argyropoulou M, Brunelle F (1992) Precocious puberty in girls: pituitary height as an index of hypothalamo-pituitary activation. J Clin Endocrinol Metab 75:1170–1172
Pelletier G, Robert F, Hardy J (1978) Identification of human anterior pituitary cells by immunoelectron microscopy. J Clin Endocrinol Metab 46:534–542
Perlman M, Schenker J, Glassman M, Ben-david M (1978) Prolonged hyperprolactinemia in preterm infants. J Clin Endocrinol Metab 47:894–897
Kiortsis D, Xydis V, Drougia AG et al (2004) The height of the pituitary in preterm infants during the first 2 years of life: an MRI study. Neuroradiology 46:224–226
Donzeau A, Bouhours-Nouet N, Fauchard M et al (2015) Birth weight is associated with the IGF-1 response to GH in children: programming of the anabolic action of GH? J Clin Endocrinol Metab 100:2972–2978
Hng T-M, Cheung NW, McLean M (2005) Growth hormone and cortisol dynamic function in relation to birth weight: a study in adult twins. J Clin Endocrinol Metab 90:2781–2786
Kopec G, Shekhawat PS, Mhanna MJ (2017) Prevalence of diabetes and obesity in association with prematurity and growth restriction. Diabetes Metab Syndr Obes 10:285–295
Luke B, Hediger M, Min S-J et al (2005) Gender mix in twins and fetal growth, length of gestation and adult cancer risk. Paediatr Perinat Epidemiol 19 Suppl 1:41–47
Mullis P-E, Tonella P (2008) Regulation of fetal growth: consequences and impact of being born small. Best Pract Res Clin Endocrinol Metab 22:173–190
Ogilvy-Stuart AL (2003) Growth hormone deficiency (GHD) from birth to 2 years of age: diagnostic specifics of GHD during the early phase of life. Horm Res 60:2–9
Becker M, Hesse V (2020) Minipuberty: why does it happen? Horm Res Paediatr 93:76–84
Carlin JB, Gurrin LC, Sterne JA et al (2005) Regression models for twin studies: a critical review. Int J Epidemiol 34:1089–1099
Möllers LS, Yousuf EI, Hamatschek C et al (2022) Metabolic-endocrine disruption due to preterm birth impacts growth, body composition, and neonatal outcome. Pediatr Res 91:1350–1360
Newbern D, Freemark M (2011) Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes 18:409–416
Wit JM, Finken MJJ, Rijken M, de Zegher F (2006) Preterm growth restraint: a paradigm that unifies intrauterine growth retardation and preterm extrauterine growth retardation and has implications for the small-for-gestational-age indication in growth hormone therapy. Pediatrics 117:e793–795
Cornblath M, Parker ML, Reisner SH et al (1965) Secretion and metabolism of growth hormone in premature and full-term infants. J Clin Endocrinol Metab 25:209–218
de Zegher F, Francois I, Boehmer AL et al (1998) Androgens and fetal growth. Horm Res 50:243–244
Hellström A, Ley D, Hansen-Pupp I et al (2016) Insulin-like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr 105:576–586
Volpe JJ (2009) The encephalopathy of prematurity–brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol 16:167–178
Loos RJ, Derom C, Eeckels R et al (2001) Length of gestation and birthweight in dizygotic twins. Lancet 358:560–561
Glinianaia SV, Magnus P, Harris JR, Tambs K (1998) Is there a consequence for fetal growth of having an unlike-sexed cohabitant in utero? Int J Epidemiol 27:657–659
James WH (2000) Why are boys more likely to be preterm than girls? Plus other related conundrums in human reproduction. Hum Reprod 15:2108–2111
Becker M, Oehler K, Partsch C-J et al (2015) Hormonal minipuberty influences the somatic development of boys but not of girls up to the age of 6 years. Clin Endocrinol (Oxf) 83:694–701
Mason KA, Schoelwer MJ, Rogol AD (2020) Androgens during infancy, childhood, and adolescence: physiology and use in clinical practice. Endocr Rev 41:bnaa003
Schmidt H, Schwarz HP (2000) Serum concentrations of LH and FSH in the healthy newborn. Eur J Endocrinol 143:213–215
Winter JS, Faiman C, Hobson WC et al (1975) Pituitary-gonadal relations in infancy. I. patterns of serum gonadotropin concentrations from birth to four years of age in man and chimpanzee. J Clin Endocrinol Metab 40:545–551
Broere-Brown ZA, Baan E, Schalekamp-Timmermans S et al (2016) Sex-specific differences in fetal and infant growth patterns: a prospective population-based cohort study. Biol Sex Differ 7:65
Acknowledgements
We would like to thank Professor Emeritus Styliani Andronikou for her valuable insights and the critical review of the manuscript.
Author information
Authors and Affiliations
Contributions
M.I.A. and D-N.K. conceived the study. A.I.D., L.G.A., V.G.X., and E.I.S. performed data management and data analyses. M.I.A. wrote the initial draft of the manuscript. V.G., L.G.A., D-N.K., and C.K.-G. provided input to the planning, execution and/or interpretation of the analysis. All authors provided critical input to the manuscript draft and approved the final version of the manuscript. M.I.A. is the guarantor of this work. The corresponding author (M.I.A.) had final responsibility to submit for publication. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Argyropoulou, M.I., Xydis, V., Astrakas, L.G. et al. Pituitary gland height evaluated with magnetic resonance imaging in premature twins: the impact of growth and sex. Pediatr Radiol 54, 787–794 (2024). https://doi.org/10.1007/s00247-024-05873-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-024-05873-0