Abstract
Background
Ongoing arthritis in children with juvenile idiopathic arthritis (JIA) can result in cartilage damage.
Objective
To study the feasibility and repeatability of T1ρ for assessing knee cartilage in JIA and also to describe T1ρ values and study correlation between T1ρ and conventional MRI scores for disease activity.
Materials and methods
Thirteen children with JIA or suspected JIA underwent 3-tesla (T) knee MRI that included conventional sequences and a T1ρ sequence. Segmentation of knee cartilage was carried out on T1ρ images. We used intraclass correlation coefficient to study the repeatability of segmentation in a subset of five children. We used the juvenile arthritis MRI scoring system to discriminate inflamed from non-inflamed knees. The Mann-Whitney U and Spearman correlation compared T1ρ between children with and without arthritis on MRI and correlated T1ρ with the juvenile arthritis MRI score.
Results
All children successfully completed the MRI examination. No images were excluded because of poor quality. Repeatability of T1ρ measurement had an intraclass correlation coefficient (ICC) of 0.99 (P<0.001). We observed no structural cartilage damage and found no differences in T1ρ between children with (n=7) and without (n=6) inflamed knees (37.8 ms vs. 31.7 ms, P=0.20). However, we observed a moderate correlation between T1ρ values and the juvenile arthritis MRI synovitis score (r=0.59, P=0.04).
Conclusion
This pilot study suggests that T1ρ is a feasible and repeatable quantitative imaging technique in children. T1ρ values were associated with the juvenile arthritis MRI synovitis score.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Juvenile idiopathic arthritis is the most common rheumatic disease in childhood [1]. Cartilage can be damaged by the autoimmune-mediated inflammation that originates in the synovial membrane. Ongoing inflammation can subsequently extend to the cartilage and result in degradation of cartilage and bone matrix. Osteochondral damage is presumably irreversible and associated with disability and decreased quality of life [2, 3]. Previous research also indicates that damaged cartilage matrix facilitates binding of synovial inflammatory cells [4]. Thus, even when inflammation subsides, a damaged matrix leaves the child at increased risk for more cartilage degradation whenever a flare occurs. Damage to the cartilage matrix is characterized by loss of proteoglycan and collagen, as demonstrated in studies focusing on rheumatoid arthritis [5,6,7,8,9]. This microstructural damage is important to recognize because intensification of anti-rheumatic treatment might prevent irreversible cartilage damage. Current imaging techniques, such as radiography, US imaging and conventional MRI, can detect structural bone damage and synovial inflammation but cannot detect microstructural damage to cartilage [10].
T1ρ is an MRI parameter that is hypothesized to quantify proteoglycan loss of the cartilage matrix in vitro [11] and in vivo [12]. T1ρ uses a weak radiofrequency pulse to lock protons in phase, which slows transverse relaxation and reduces the effect of dipolar interactions. As a result, the measured relaxation of protons can be attributed to time-constant T1ρ. Earlier studies in osteoarthritis and rheumatoid arthritis [12,13,14,15,16] showed that T1ρ values increase as a consequence of decreased proteoglycan content. Cartilage integrity was studied once in pediatric patients using T1ρ: knee cartilage of 10 healthy children was assessed and a mean T1ρ value of 76.6 ms was reported [17]. Cartilage in children is distinct from articular cartilage in adults. Postnatally, chondrocytes are small and there is a scant matrix without a distinct zonal orientation [18]. Animal studies demonstrated that proteoglycan content is highest postnatally, with a gradual decrease in proteoglycans during aging, while collagen content increases and collagen fibers show increasing isotropy, resulting in development of a zonal organization of the cartilage [19,20,21].
The primary aim of this pilot study was to evaluate feasibility (motion artefacts and patient comfort) of T1ρ knee cartilage imaging in children with juvenile idiopathic arthritis; secondarily we studied repeatability of T1ρ values derived by manual cartilage segmentation. Moreover, we explored T1ρ values in juvenile idiopathic arthritis by comparing T1ρ values with conventional MRI scores using the juvenile arthritis MRI scoring system [22].
Materials and methods
Patients
In this pilot study, we included all consecutive pediatric patients who underwent MRI of the knee between April 2016 and August 2016 in the Academic Medical Center/Univerisity of Amsterdam, Amsterdam, The Netherlands. Patients visited one of the outpatient clinics of three tertiary pediatric rheumatology centers the Academic Medical Center/Univerisity of Amsterdam, OLVG hospital and Reade, all in Amsterdam, the Netherlands. Patients were clinically assessed by one of our pediatric rheumatologists J.M. van den Berg, D. Schonenberg-Meinema, A. Nassar-Sheikh Rashid and K.M. Dolman. Clinical characteristics consisted of age, height, weight, body mass index (adjusted for age and gender using body mass index percentiles and body mass index z-scores [23], calculated using the pediatric z-score calculator from the Children’s Hospital of Philadelphia [24]); a global assessment of disease activity measured on a 0–100 visual analogue scale by the physician; and an 84-joint count evaluating the number of actively inflamed joints. Laboratory tests included the erythrocyte sedimentation rate, which is the most widely used laboratory marker for juvenile idiopathic arthritis-related inflammation [25], and C-reactive protein.
Inclusion criteria for this pilot study were (1) clinically active arthritis (defined as joint swelling or limitation of motion, with pain or tenderness to palpation) involving at least one knee in children with juvenile idiopathic arthritis or suspected new-onset juvenile idiopathic arthritis; or (2) follow-up of children with juvenile idiopathic arthritis with clinically inactive disease who had a history of clinically evident arthritis in at least one knee.
Exclusion criteria were intra-articular corticosteroid injection within the last 6 months, the need for anesthesia during MRI examination and general contraindications for MRI. Written informed consent was obtained from all parents and, if the patient was 12 years or older, written informed consent was also obtained from the patient, as prescribed by local ethics regulations.
Magnetic resonance imaging protocol
Knee MR images were obtained using a 3.0-T magnet (Ingenia; Philips Medical Systems, Best, the Netherlands) and comprised all sequences of our standard juvenile idiopathic arthritis MRI protocol and in addition a sagittal T1ρ, which was acquired before administration of contrast agent (Table 1). To measure T1ρ we used a B0/B1-compensated T1ρ-prepared 3-D gradient echo sequence. Each spin lock pulse consisted of two continuous radiofrequency pulses with opposite phase to compensate for B1 variations. A 180° refocusing pulse was placed between the two continuous radiofrequency pulses to compensate for B0 inhomogeneities [26, 27]. We repeated the sequence with five spin lock times (5 ms, 10 ms, 20 ms, 40 ms and 70 ms), spin lock frequency of 400 Hz and imaging acceleration sensitivity encoding factor 2. The acquisition time for a single spin lock time was 1 min 51 s, resulting in a total acquisition time of 9 min 15 s for the complete T1ρ scan. Scans were made in non-loading conditions with children placed in supine position with the knee in the center of the scanner bore. A dedicated 16-channel transmit/receive knee coil was used. To standardize the influence of potential biomechanical load [28, 29], we kept loading differences prior to the MRI examination to a minimum by preparing all patients equally: 1 h before MRI, children were invited in the hospital for intravenous cannula placement, and subsequently they were taken to the radiology department by one of our researchers using the elevator. All children walked a similar distance of maximum 500 m and did not participate in other knee-loading activities in the hour prior to the MRI.
Image analysis
The conventional MR image sets were scored by an experienced reader R. Hemke (RH), with 7 years’ experience in musculoskeletal radiology) who was blinded to the clinical history of the patients. The reader scored the presence and extent of disease activity and osteochondral damage using the validated juvenile arthritis MRI scoring system, which has been described in detail [22].
T1ρ post-processing
After image acquisition the T1ρ images acquired at longer spin lock times were registered to the T1ρ images of the corresponding shortest spin lock time (5 ms) using an affine registration pipeline implemented in elastix [30]. This was done to correct for small subject motion occurring between the different scans. The T1ρ relaxation maps were obtained by fitting the signal intensities of the registered images to a three-parameters exponential decay model in a pixel-by-pixel fashion.
Cartilage segmentation
The total articular cartilage of the knee was manually segmented into eight regions of interest by an experienced reader (RH, with 7 years’ experience in musculoskeletal radiology) on the T1ρ images acquired with spin lock time of 5 ms using ITK-SNAP [31]. Segmentation was performed using the conventional axial, sagittal and coronal images to ensure accurate segmentation of the cartilage. The regions of interest reflected eight anatomical cartilage regions following the juvenile arthritis MRI scoring system [22]. T1ρ values in milliseconds were extracted per region of interest and then combined, resulting in mean T1ρ values of the total articular cartilage per patient. Moreover, the regions of interest were sub-grouped into weight-bearing cartilage (medial and lateral tibial plateau, medial and lateral weight-bearing femur) or non-weight-bearing cartilage (medial and lateral patella, medial and lateral anterior part of condyle) resulting in mean T1ρ values for the weight-bearing cartilage and non-weight-bearing cartilage.
Reliability of repeated cartilage segmentation
To analyze the repeatability of the delineation of the articular knee cartilage on T1ρ, the same reader [blinded] performed the delineation process a second time in five randomly selected patients, 4 months after the first delineations.
Statistical analysis
All analyses were carried out using SPSS Statistics version 24.0 (IBM Corp., Armonk, NY). We studied normality of the data and whether the data had a non-Gaussian distribution; medians and interquartile range were reported and non-parametric tests were used for analysis. A P-value <0.05 was considered to indicate a statistically significant difference. First, descriptive characteristics of the patients were reported. Second, we studied the intra-reader reliability in five children who were segmented twice by the same reader using the intraclass correlation coefficient in a two-way mixed model with single measures. To further gain insight into T1ρ differences between the first and second segmentation, we created a Bland–Altman plot and calculated and the coefficient of repeatability (1.96 × standard deviation of the differences) [32]. Next, we studied the difference between total mean T1ρ values and individual region-of-interest T1ρ values in MRI-active disease (defined as children with a juvenile arthritis MRI score ≥1) as compared to MRI-inactive disease (defined as children with a juvenile arthritis MRI score of 0) and we studied the difference in T1ρ values between weight-bearing and non-weight-bearing cartilage, all using Mann–Whitney U test. Subsequently, we studied correlation between the T1ρ value and disease activity parameters (juvenile arthritis MRI score, erythrocyte sedimentation rate, number of actively inflamed joints) with a Spearman ρ test. Last, we studied correlation between T1ρ values and age and body mass index of the patients with a Spearman ρ test.
Results
Patients
From April 2016 to August 2016, we included 13 children (9/13 or 69% girls; median age 13.7 years, interquartile range [IQR] 11.6–15.9 years). Demographic and clinical characteristics can be found in Table 2. In five children with suspected new-onset juvenile idiopathic arthritis at the date of MRI, the diagnosis juvenile idiopathic arthritis was not confirmed. They were diagnosed with reactive arthritis, panuveitis with arthralgia, hypermobility and pain syndrome (n=2), respectively.
Feasibility
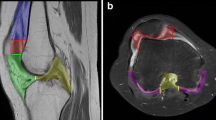
All 13 children underwent T1ρ MRI scanning without discomfort and the total MR protocol was acquired without any problems. No movement artifacts were observed and all acquired T1ρ images were of sufficient quality for post-processing. Figure 1 depicts conventional and T1ρ MR imaging examples.
Two T1ρ MR imaging examples in children from this study. a, b T1ρ overlay image (a) of a 10-year-old boy with oligo-articular juvenile idiopathic arthritis who had a swollen and warm right knee with slight limitation of motion on examination. On the image, higher T1ρ values are observed as compared to the child depicted in (b). Especially the cartilage in the patellofemoral joint demonstrates high T1ρ values (arrow). In this boy, synovial inflammation was observed on MR imaging in the patellofemoral synovium and around the cruciate ligaments. T1ρ overlay image (b) of a 13-year-old girl who presented with knee pain. She was suspected of having juvenile idiopathic arthritis. On examination, minor swelling of the knee was found. The conventional MRI showed no synovial inflammation in the knee. T1ρ values are markedly lower as compared to (a). Note the higher relative contribution of purple and blue in the cartilage overlay. After clinical, laboratory and imaging evaluation she was diagnosed with a functional pain syndrome
Repeatability of segmentation-derived T1ρ values
To study repeatability of the T1ρ values that were derived from manual knee cartilage segmentations, the reader repeated the segmentation in five randomly selected children 4 months after the first segmentations. The intraclass correlation coefficient of the T1ρ values was 0.99 (95% confidence interval [CI] 0.99–1.00, P-value <0.001). For further analysis, we created a Bland–Altman plot (Fig. 2), which showed small differences between measurements. Coefficient of repeatability was 0.51 ms, which is a difference of <2% from the mean T1ρ value.
T1ρ values in magnetic resonance imaging (MRI)-active versus MRI-inactive disease
Morphologic cartilage damage (cartilage erosion or thinning of the cartilage) was not seen in any of the children on the conventional MR sequences. Children without inflammation on MRI (juvenile arthritis MRI score of 0, n=6) had a median T1ρ value of 31.7 ms (IQR 29.5–33.6), while children with inflammation on MRI (juvenile arthritis MRI score of 1 or higher, n=7) had a median T1ρ value of 37.8 ms (IQR 31.0–44.3; Fig. 3). This difference was not statistically significant (P=0.20). T1ρ values of the eight individual regions of interest are presented in Table 3 and Fig. 4. T1ρ values of the cartilage of the lateral patella were significantly lower in children without inflammation on MRI (35.4 ms, IQR 32.5–38.1) as opposed to children with inflammation on MRI (40.4 ms, IQR 34.4–44.1), P=0.046.
Boxplots of T1ρ values by MRI-inactive disease (left in the boxplot, juvenile arthritis MRI score –) and MRI-active disease (right in the boxplot, juvenile arthritis MRI score +). a–h Readings within the medial tibial plateau (a), medial patella (b), lateral tibial plateau (c), lateral patella (d), medial weight-bearing femur (e), medial femoral condyle (f), lateral weight-bearing femur (g) and lateral femoral condyle (h). Consistently, all regions of interest show lower T1ρ values for children with MRI-inactive disease. Significant difference was only seen in the lateral patella (P=0.046). JAMRIS juvenile arthritis MRI score
Weight-bearing cartilage versus non-weight-bearing cartilage
Overall, weight-bearing cartilage had a significantly lower T1ρ value compared to non-weight-bearing cartilage, with T1ρ values for weight-bearing cartilage of 29.5 ms (IQR 27.6–36.0) and 34.8 ms (IQR 33.7–40.4) for non-weight-bearing cartilage, P-value=0.04.
Correlation between T1ρ and disease activity parameters, age and body mass index
The Spearman correlation coefficient between T1ρ and the juvenile arthritis MRI score was 0.59 (P=0.04). When the six children with a juvenile arthritis MRI score of 0 were excluded, correlation between T1ρ and the juvenile arthritis MRI score was 0.85 (P=0.02; Fig. 5). T1ρ and erythrocyte sedimentation rate had a correlation coefficient of 0.66 with a P-value of 0.01 (Fig. 5). No significant correlation was observed between T1ρ and the number of actively inflamed joints (correlation coefficient 0.15, P-value 0.62). Also, patient age and body mass index percentile were not correlated with the T1ρ value (correlation coefficient −0.5, P-value 0.09 and −0.07, P-value 0.81, respectively).
Plot shows correlation between T1ρ and disease activity parameters. a Correlation between T1ρ values and the juvenile arthritis MRI score. b Correlation between T1ρ values and erythrocyte sedimentation rate. The correlation coefficient, r, and associated P-values are given in the plot. In (a) both the correlation for all children (closed and open circles) and the correlation for only those with juvenile arthritis MRI≥1 score (closed circles) are given. P<0.05 is significant
Discussion
In this pilot study, we show the feasibility of T1ρ for assessing knee cartilage integrity in children with juvenile idiopathic arthritis. All 13 children in the study underwent the T1ρ acquisition protocol without discomfort and all images were of sufficient quality. None of the 13 children showed structural cartilage damage on conventional MRI. We found excellent repeatability for derivation of T1ρ values using manual cartilage segmentations on the T1ρ images.
Concerning the assessment of cartilage integrity, we found high correlation between T1ρ values and the juvenile arthritis MRI score, an MRI-based disease activity score, in the seven children who had inflammation in the knee, but we also observed that T1ρ values in children with actively inflamed knees were not different compared to T1ρ values in those with non-inflamed knees. Nevertheless, cartilage of the lateral patellar region of interest demonstrated significantly higher T1ρ values as compared to cartilage in children without knee inflammation on MRI. The patellar cartilage borders the patellofemoral synovium, which is often affected if knee arthritis is present [33]. Correspondingly, in our study, 4 of the 7 children with active arthritis indeed showed inflamed patellofemoral synovium. Thus, we hypothesize that increased T1ρ values might represent pre-erosive microstructural damage to proteoglycans and collagen in the cartilage matrix that is not visualized using conventional MR sequences.
Several studies confirmed that T1ρ values can be used to detect macrostructural and microstructural damage to cartilage in osteoarthritis [34,35,36]. In rheumatoid arthritis, the use of T1ρ was first described in a study involving five people with rheumatoid arthritis [12]. In this study of cartilage specimens after total knee arthroplasty, T1ρ values correlated with histological Safranin-O staining and macroscopic grade of severity of cartilage degeneration. In another study, radiocarpal cartilage was evaluated in a 3-month follow-up study of nine people with rheumatoid arthritis who used anti-rheumatic medication [37]. T1ρ values correlated with treatment response, showing the potential of T1ρ to measure changes in cartilage structure following treatment. Our results seem comparable with the findings in both osteoarthritis and rheumatoid arthritis [10, 12, 37] in which people with more severe disease activity were found to have higher T1ρ values. When comparing the absolute T1ρ values, we found (lowest-to-highest) 31 ms to 55 ms. Values in people with rheumatoid arthritis have been found to be 38–62 ms [12], and values in healthy pediatric patients 66–77 ms [17]. Note our values are somewhat lower. For the first comparison, this is probably attributable to the more severely affected cartilage in these children with rheumatoid arthritis who were scheduled for total knee arthroplasty. Another factor to take into account when comparing results from different studies is the spin lock frequency because T1ρ values are higher at increased spin lock frequency. Our scans were acquired at lower frequency (400 Hz) than the scans of people with rheumatoid arthritis and healthy pediatric subjects (both acquired at 500 Hz) [12, 17].
We found a correlation between the juvenile arthritis MRI score and T1ρ values as well as erythrocyte sedimentation rate and T1ρ values. This supports the hypothesis that inflammation in the knee negatively affects the cartilage. We could not confirm our hypothesis that increasing age, and thus lower proteoglycan content, leads to lower T1ρ values because we observed no correlation between age and T1ρ values in this small cohort. However, this could be influenced by the age dispersion in our cohort because all but one child was older than 10 years.
Concerning body mass index, literature shows contradictory results. A recent study found correlation between body mass index and T1ρ in the knee [38] while others decline a relation between body mass index and T1ρ values in hip cartilage and intervertebral disc cartilage, respectively [39, 40]. In our study, body mass index was not correlated to T1ρ values. It should be noted that our cohort consisted of mainly non-obese adolescents, thus we cannot exclude that age and body mass index could influence T1ρ values in, for example, a 4-year-old or heavily obese child.
Limitations of this study are that none of the children had structural cartilage damage on MRI, hence it was not possible to examine the T1ρ value in actual erosive cartilage damage. Moreover, histochemical proof of the hypothesized pre-erosive microstructural proteoglycan loss in the cartilage is lacking. However, obtaining cartilage specimens using biopsy is not feasible because this would harm the joints of these children. Furthermore, our T1ρ experiments were performed at a spin lock frequency of 400 Hz because of specific absorption rate limitations and the need to keep the acquisition time short enough. Therefore, the used spin lock preparation did not completely remove all the contributions of dipolar interactions to the relaxation process. As a consequence, our readout is not completely specific to proteoglycan content and likely also reflects changes in the collagen matrix, such as degradation or swelling. Additional studies are needed in order to decouple the two contributions and gain more insight into the biochemical modifications induced by the disease. Another limitation is our segmentation. Although the cartilage segmentations were performed meticulously by an experienced reader, we cannot rule out that the cartilage–bone and cartilage–soft-tissue boundaries were imperfect. This could have influenced our results, especially if possible fluid pixels from joint effusion were wrongly included in the segmentation. To prevent this, we used three imaging planes when drawing the segmentations. Second, we performed our segmentation on full-thickness articular cartilage. Because cartilage has a zonal orientation, it would be interesting to subdivide the cartilage into a superficial and deep layer and study spatial variation in more detail. This could, for example, be performed using a normalization procedure to correct for different cartilage thicknesses in children, as has been done by authors studying cartilage with T2 relaxation time mapping in healthy children and children with juvenile idiopathic arthritis [41,42,43,44]. However, because our primary goal was to evaluate the feasibility of T1ρ, we did not perform such in-depth analyses of the cartilage; nevertheless, we would recommend a zonal analysis of the cartilage in studies that include a bigger sample of patients. Last, the small patient sample itself is considered a limitation and further work should focus on inclusion of more patients to validate the results of our pilot study. When more patients are included, the likelihood of scanning patients with structural cartilage damage would increase, which is important to affirm the assumption that higher T1ρ values are seen in structurally abnormal cartilage as seen on conventional, qualitative MRI.
Conclusion
There was acceptable feasibility and repeatability of T1ρ for assessing knee cartilage in our sample. We found some association between T1ρ values and erythrocyte sedimentation rate and the juvenile arthritis MRI score. In further studies, inclusion of more children — including children with erosive cartilage damage in the knee — is warranted to confirm the preliminary findings of this pilot study.
References
Ravelli A, Martini A (2007) Juvenile idiopathic arthritis. Lancet 369:767–778
Susic GZ, Stojanovic RM, Pejnovic NN et al (2011) Analysis of disease activity, functional disability and articular damage in patients with juvenile idiopathic arthritis: a prospective outcome study. Clin Exp Rheumatol 29:337–344
Aletaha D, Funovits J, Smolen JS (2011) Physical disability in rheumatoid arthritis is associated with cartilage damage rather than bone destruction. Ann Rheum Dis 70:733–739
Pap T, Korb-Pap A (2015) Cartilage damage in osteoarthritis and rheumatoid arthritis — two unequal siblings. Nat Rev Rheumatol 11:606–615
Korb-Pap A, Stratis A, Muhlenberg K et al (2012) Early structural changes in cartilage and bone are required for the attachment and invasion of inflamed synovial tissue during destructive inflammatory arthritis. Ann Rheum Dis 71:1004–1011
Schleich C, Muller-Lutz A, Sewerin P et al (2015) Intra-individual assessment of inflammatory severity and cartilage composition of finger joints in rheumatoid arthritis. Skelet Radiol 44:513–518
Renner N, Kronke G, Rech J et al (2014) Anti-citrullinated protein antibody positivity correlates with cartilage damage and proteoglycan levels in patients with rheumatoid arthritis in the hand joints. Arthritis Rheumatol 66:3283–3288
Martel-Pelletier J, Welsch DJ, Pelletier JP (2001) Metalloproteases and inhibitors in arthritis diseases. Best Pract Res Clin Rheumatol 15:805–829
Herz B, Albrecht A, Englbrecht M et al (2014) Osteitis and synovitis, but not bone erosion, is associated with proteoglycan loss and microstructure damage in the cartilage of patients with rheumatoid arthritis. Ann Rheum Dis 73:1101–1106
Wang L, Regatte RR (2015) T(1)rho MRI of human musculoskeletal system. J Magn Reson Imaging 41:586–600
Akella SV, Regatte RR, Gougoutas AJ et al (2001) Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med 46:419–423
Tsushima H, Okazaki K, Takayama Y et al (2012) Evaluation of cartilage degradation in arthritis using T1rho magnetic resonance imaging mapping. Rheumatol Int 32:2867–2875
Takayama Y, Hatakenaka M, Tsushima H et al (2013) T1rho is superior to T2 mapping for the evaluation of articular cartilage denaturalization with osteoarthritis: radiological-pathological correlation after total knee arthroplasty. Eur J Radiol 82:e192–e198
Duvvuri U, Kudchodkar S, Reddy R, Leigh JS (2002) T(1rho) relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthr Cartil 10:838–844
Regatte RR, Akella SV, Borthakur A et al (2002) Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol 9:1388–1394
Regatte RR, Akella SV, Wheaton AJ et al (2004) 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol 11:741–749
Cobb JG, Kan JH, Gore JC (2013) T1rho mapping of pediatric epiphyseal and articular cartilage in the knee. J Magn Reson Imaging 38:299–305
Decker RS (2017) Articular cartilage and joint development from embryogenesis to adulthood. Semin Cell Dev Biol 62:50–56
Brama PA, Holopainen J, van Weeren PR et al (2009) Influence of exercise and joint topography on depth-related spatial distribution of proteoglycan and collagen content in immature equine articular cartilage. Equine Vet J 41:557–563
Hyttinen MM, Holopainen J, van Weeren PR et al (2009) Changes in collagen fibril network organization and proteoglycan distribution in equine articular cartilage during maturation and growth. J Anat 215:584–591
Julkunen P, Halmesmaki EP, Iivarinen J et al (2010) Effects of growth and exercise on composition, structural maturation and appearance of osteoarthritis in articular cartilage of hamsters. J Anat 217:262–274
Hemke R, van Rossum MA, van Veenendaal M et al (2013) Reliability and responsiveness of the juvenile arthritis MRI scoring (JAMRIS) system for the knee. Eur Radiol 23:1075–1083
Cole TJ, Faith MS, Pietrobelli A, Heo M (2005) What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr 59:419–425
Children’s Hospital of Philadelphia (2019) Pediatric Z-score calculator. https://zscore.research.chop.edu/index.php. Accessed 14 Sept 2019
Consolaro A, Ruperto N, Bazso A et al (2009) Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 61:658–666
Van Oorschot JW, El Aidi H, Jansen of Lorkeers SJ et al (2014) Endogenous assessment of chronic myocardial infarction with T1rho mapping in patients. J Cardiovasc Magn Reson 16:104
Hectors SJ, Jacobs I, Strijkers GJ, Nicolay K (2014) Multiparametric MRI analysis for the identification of high intensity focused ultrasound-treated tumor tissue. PLoS One 9:e99936
Subburaj K, Kumar D, Souza RB et al (2012) The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am J Sports Med 40:2134–2141
Souza RB, Kumar D, Calixto N et al (2014) Response of knee cartilage T1rho and T2 relaxation times to in vivo mechanical loading in individuals with and without knee osteoarthritis. Osteoarthr Cartil 22:1367–1376
Klein S, Staring M, Murphy K et al (2010) elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 29:196–205
Yushkevich PA, Piven J, Hazlett HC et al (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31:1116–1128
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8:135–160
Nusman CM, Hemke R, Schonenberg D et al (2014) Distribution pattern of MRI abnormalities within the knee and wrist of juvenile idiopathic arthritis patients: signature of disease activity. AJR Am J Roentgenol 202:W439–W446
Pedoia V, Gallo MC, Souza RB, Majumdar S (2017) Longitudinal study using voxel-based relaxometry: association between cartilage T1rho and T2 and patient reported outcome changes in hip osteoarthritis. J Magn Reson Imaging 45:1523–1533
Gallo MC, Wyatt C, Pedoia V et al (2016) T1rho and T2 relaxation times are associated with progression of hip osteoarthritis. Osteoarthr Cartil 24:1399–1407
Wyatt C, Kumar D, Subburaj K et al (2015) Cartilage T1rho and T2 relaxation times in patients with mild-to-moderate radiographic hip osteoarthritis. Arthritis Rheumatol 67:1548–1556
Ku E, Pedoia V, Tanaka M et al (2017) Evaluating radiocarpal cartilage matrix changes 3-months after anti-TNF treatment for rheumatoid arthritis using MR T1rho imaging. J Magn Reson Imaging 45:1514–1522
Collins AT, Kulvaranon ML, Utturkar GM et al (2017) In vivo biochemical and biomechanical properties of cartilage are affected by obesity. Osteoarthr Cartil 25:S112–S113
Nemeth A, Di Marco L, Boutitie F et al (2018) Reproducibility of in vivo magnetic resonance imaging T1 rho and T2 relaxation time measurements of hip cartilage at 3.0T in healthy volunteers. J Magn Reson Imaging 47:1022–1033
Zobel BB, Vadala G, Del Vescovo R et al (2012) T1rho magnetic resonance imaging quantification of early lumbar intervertebral disc degeneration in healthy young adults. Spine 37:1224–1230
Dardzinski BJ, Laor T, Schmithorst VJ et al (2002) Mapping T2 relaxation time in the pediatric knee: feasibility with a clinical 1.5-T MR imaging system. Radiology 225:233–239
Kight AC, Dardzinski BJ, Laor T, Graham TB (2004) Magnetic resonance imaging evaluation of the effects of juvenile rheumatoid arthritis on distal femoral weight-bearing cartilage. Arthritis Rheum 50:901–905
Kim HK, Laor T, Graham TB et al (2010) T2 relaxation time changes in distal femoral articular cartilage in children with juvenile idiopathic arthritis: a 3-year longitudinal study. AJR Am J Roentgenol 195:1021–1025
Kim HK, Shiraj S, Anton CG et al (2014) Age and sex dependency of cartilage T2 relaxation time mapping in MRI of children and adolescents. AJR Am J Roentgenol 202:626–632
Acknowledgments
We acknowledge our colleagues for their valuable assistance in conducting this study: E.C. van Gulik, MD, D. Schonenberg-Meinema, MD, A. Nassar-Sheikh Rashid, MD, K.M. Dolman, MD, PhD. A.M.B. received an MD/PhD scholarship from the Academic Medical Center (AMC)/University of Amsterdam, the Netherlands.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Barendregt, A.M., Mazzoli, V., van den Berg, J.M. et al. T1ρ-mapping for assessing knee joint cartilage in children with juvenile idiopathic arthritis — feasibility and repeatability. Pediatr Radiol 50, 371–379 (2020). https://doi.org/10.1007/s00247-019-04557-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-019-04557-4