Abstract
Background
Detection of cardiac fibrosis based on endogenous magnetic resonance (MR) characteristics of the myocardium would yield a measurement that can provide quantitative information, is independent of contrast agent concentration, renal function and timing. In ex vivo myocardial infarction (MI) tissue, it has been shown that a significantly higher T1ρ is found in the MI region, and studies in animal models of chronic MI showed the first in vivo evidence for the ability to detect myocardial fibrosis with native T1ρ-mapping. In this study we aimed to translate and validate T1ρ-mapping for endogenous detection of chronic MI in patients.
Methods
We first performed a study in a porcine animal model of chronic MI to validate the implementation of T1ρ-mapping on a clinical cardiovascular MR scanner and studied the correlation with histology. Subsequently a clinical protocol was developed, to assess the feasibility of scar tissue detection with native T1ρ-mapping in patients (n = 21) with chronic MI, and correlated with gold standard late gadolinium enhancement (LGE) CMR. Four T1ρ-weighted images were acquired using a spin-lock preparation pulse with varying duration (0, 13, 27, 45 ms) and an amplitude of 750 Hz, and a T1ρ-map was calculated. The resulting T1ρ-maps and LGE images were scored qualitatively for the presence and extent of myocardial scarring using the 17-segment AHA model.
Results
In the animal model (n = 9) a significantly higher T1ρ relaxation time was found in the infarct region (61 ± 11 ms), compared to healthy remote myocardium (36 ± 4 ms) . In patients a higher T1ρ relaxation time (79 ± 11 ms) was found in the infarct region than in remote myocardium (54 ± 6 ms). Overlap in the scoring of scar tissue on LGE images and T1ρ-maps was 74%.
Conclusion
We have shown the feasibility of native T1ρ-mapping for detection of infarct area in patients with a chronic myocardial infarction. In the near future, improvements on the T1ρ -mapping sequence could provide a higher sensitivity and specificity. This endogenous method could be an alternative for LGE imaging, and provide additional quantitative information on myocardial tissue characteristics.
Similar content being viewed by others
Background
Detection of the location and extent of myocardial scarring is important for the prognosis of patients with myocardial remodeling [1]. Post-infarct formation of myocardial fibrosis can lead to adverse cardiac remodeling and subsequently, to heart failure. The current in vivo reference standard for detection of myocardial scar tissue is late gadolinium enhancement (LGE), where the prolonged retention of gadolinium contrast agent (CA) in regions of myocardial fibrosis results in increased signal intensity on T1 weighted cardiovascular magnetic resonance (CMR) images. The presence and extent of LGE CMR carries important prognostic value as has been demonstrated in several ischemic and non-ischemic cardiomyopathies [2]–[4].
LGE is a validated method with a high sensitivity to discriminate infarcted from healthy myocardium. However, to guide and evaluate medical treatment, more information is needed about the heterogeneity of myocardial damage associated with diverse cardiac disease processes, as this damage is the substrate of arrhythmias, and a possible target for CMR-guided arrhythmia ablation. In order to provide this information, a shift from mere visualization to quantification of myocardial fibrosis is needed. Furthermore, a truly noninvasive method based on endogenous contrast without the requirement for an exogenous contrast agent would be preferable, since gadolinium enhanced CMR with some agents is off-label use [5]. Allergic reactions after intravenous administration of gadolinium-based contrast agents are very rare but potentially life threatening [6],[7], and the method cannot be applied in patients with severe renal failure [8]. A quantitative method capable of detecting myocardial fibrosis based on endogenous MR characteristics of the myocardium could, therefore, be a valuable tool, complementary to LGE [9].
In the field of orthopedics, the MR relaxation parameter T1 in the rotating frame (T1ρ) is well established as measure for the collagen content in cartilage [10],[11]. Recent studies have hypothesized that this method may be applicable to directly image collagen in the heart, and therefore would be a promising candidate for detection of chronic myocardial infarction [12],[13]. The T1ρ relaxation time describes relaxation while the magnetization is in the rotating frame, in the presence of a so-called spin-lock pulse. A spin-lock pulse is a low amplitude radiofrequency (RF) pulse on-resonance with the precessing transverse magnetization. By acquiring images with varying T1ρ weighting, a so-called T1ρ-map can be calculated.
In ex vivo MI tissue, it has been shown that the T1ρ relaxation time is sensitive to changes in macromolecular content, and that a significantly higher T1ρ is found in the MI region [12]. Studies in animal models of chronic MI showed the first in vivo evidence for the ability to detect myocardial fibrosis with T1ρ-mapping [14],[15]. Furthermore it has been shown that the addition of T1ρ weighting to a conventional gradient echo sequence improves the contrast between acutely infarcted and noninfarcted myocardium in patients in an acute (63 ± 40 hours) myocardial infarction [13]. However, this method has not yet been reported for assessment of chronic MI in humans.
The aim of the current study is to explore the potential of T1ρ-mapping to detect fibrosis in patients with chronic ischemic heart disease on a standard clinical MR scanner. Therefore, we first performed a study in a porcine animal model of chronic MI to reproduce initial results in literature and to validate the implementation of the technique. In a second study the method was translated to a clinical protocol, and the feasibility of detecting scar tissue with native T1ρ-mapping in patients with chronic MI was assessed and correlated with LGE CMR.
Methods
Animal study
Animal model
All in vivo animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources. Experiments were approved by the Animal Experimentation Committee of the University Medical Center Utrecht.
Nineteen Dalland landrace pigs (69 ± 5 kg) underwent a 90-minute percutaneous balloon occlusion of the midpart of the left anterior descending (LAD) coronary artery, followed by reperfusion. 16 animals survived the LAD occlusion.
CMR method: in vivo animal study
Eight weeks after myocardial infarction, an in vivo CMR was performed under anesthesia on a clinical 3 T MR scanner (Achieva TX, Software release 3.2.1, Philips Healthcare, Best, The Netherlands), using a commercially available 32-channel cardiac receive coil.
T1ρ-mapping was performed using a 3D, T1ρ -prepared, multi-shot gradient echo sequence. T1ρ –preparation was performed using a spin-lock pulse (Figure 1) consisting of 2 continuous RF pulses with opposite phase to compensate for B1 variations, and a refocusing pulse between the spin-locking halves to compensate for B0 errors [16],[17]. The amplitude of the spin-lock pulse was set to 500 Hz (11.7 μT), and five images with different spin-lock (SL) preparation times were acquired (SL = 1, 10, 20, 30, 40 ms). Other parameters were: bandwidth/pixel = 287 Hz, TE/TR = 2.6/5.3 ms, resolution = 1.5 × 1.5 mm2, slice thickness = 6 mm, FOV = 336 × 336 mm2, flip angle = 8 degrees, 8 TFE shots, number of signals averaged (NSA) =2, shot interval = 2 heart beats.
After the T1ρ-mapping 0.2 ml/kg gadobutrol (Gadovist, Bayer Healthcare, Berlin, Germany) was injected and LGE CMR was performed 15 minutes after injection. A look-locker scout sequence was performed to optimize nulling of the remote myocardium. LGE imaging parameters were: TI = 200–250 ms, TE/TR = 1.5/4.7 ms, resolution = 1.5 × 1.5 mm2, slice thickness = 6 mm, FOV = 300 × 300 mm2, flip angle = 25 degrees, 63 TFE shots).
Histology
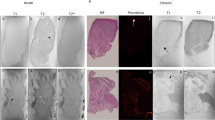
After the CMR examination the animals were sacrificed, and triphenyltetrazolium chloride (TTC) staining of serially sectioned heart slices was performed (slice thickness = 2 cm). Heart tissue from infarct, borderzone and remote areas were fixated in formalin, embedded in parafine, and 5 μm slides were stained with picrosirius red for fibrosis quantification.
CMR methods: Ex vivo animal study
From 5 pigs the third sectioned slice from the apex, was scanned before histology on a clinical 1.5 T MR scanner (Achieva, Philips Healthcare, Best, the Netherlands) in an 8 channel head coil. T1ρ dispersion was measured using a 3D, T1ρ -prepared, multi-shot gradient echo sequence. A T1ρ map was calculated by acquiring five images with different spin-lock preparation time (SL = 1, 10, 20, 30, 40 ms). The amplitude (B1) of the spin-lock pulse was varied, to assess the T1ρ dispersion (B1 = 0, 100, 200, 500, 750, 925 Hz). The maximum B1 of the spin-lock pulse was limited by the maximum B1 achievable by the transmit coil. Other imaging parameters were: bandwidth/pixel = 889 Hz, TE/TR = 1.7/3.9 ms, resolution = 1 × 1 mm2, slice thickness = 1 mm, 25 slices, FOV = 180 × 180 mm2, flip angle = 8 degrees, 64 TFE shots, NSA =1 and shot interval = 3000 ms.
Patient study
Patients
Twenty-one patients with a first reperfused ST-segment elevation MI underwent a CMR between 3 and 12 months after the acute event (Table 1). Written informed consent was obtained from all participants, and the study was approved by the local Ethical Review Board of the University Medical Center Utrecht. Five healthy young control subjects (5 male, age 25 ± 3 years) were scanned to confirm measurement of the remote tissue.
CMR methods
All subjects were imaged on a clinical 1.5 T MR scanner (Achieva, Philips Healthcare, Best, the Netherlands), using a 5-channel cardiac receive coil. T1ρ-mapping was performed using a 2D T1ρ-prepared balanced steady-state free precession (SSFP) gradient echo sequence, using the same spin-lock preparation scheme as in the animal study (Figure 1). In the first three patients a multi-shot gradient echo readout instead of a balanced SSFP readout was used, due to further optimization of the protocol. The amplitude of the spin-lock pulse was set to 750 Hz (17.6 μT), and four images with different spin-lock preparation times were acquired (SL = 1, 13, 27, 45 ms). Other parameters were: bandwidth/pixel = 530 Hz, TE/TR = 1.94/3.9 ms, resolution = 1.5 × 1.65 mm, slice thickness = 6 mm, slice gap = 4 mm, FOV = 288 × 288 mm2, flip angle = 50 degrees, 2 TFE shots, NSA = 2, SENSE acceleration = 1.5, shot interval = 3 heart beats. In all participants, the T1ρ-mapping was performed in 8 short axis slices, acquired in late diastole during expiration breath holds, covering the heart from apex to base.
In the patients, LGE CMR was performed in the same 8 short axis slices, 15 minutes after intravenous contrast injection (0.2 ml/kg contrast agent (Gadovist, Bayer Healthcare). A look-locker scout was performed to optimize nulling of the remote myocardium. LGE imaging parameters were: TI = 300–340 ms, TE/TR = 3.5/7.1 ms, resolution = 1.5 × 1.65 mm2, slice thickness = 8 mm, slice gap = 4 mm, FOV = 288 × 288 mm2, flip angle = 25 degrees, 5 shots).
Post processing and image analysis
For both the animal and the patient studies, T1ρ-maps were calculated by pixelwise fitting of a mono-exponential decay function in Matlab (Release 2012a, Mathworks, Massachusetts, United States).
The LGE images and T1ρ maps were scored by a radiologist (TL) with over 10 years experience in the evaluation of CMR. Images were scored for image quality, location of the infarct, and transmurality.
The LGE images and T1ρ maps were scored separately using the 17 segments AHA-model [18]. The T1ρ-maps were scored two times by the observer. The first time the observer was untrained, and blinded for clinical characteristics, results of conventional LGE imaging and the LGE images were not shown. The second time the observer was trained for looking at T1ρ-maps, and the LGE images were shown along with the T1ρ-maps, but the observer was blinded for clinical characteristics and the results on the scoring of the LGE images.
Segmentation of infarct and remote myocardium was based on the 2SD segmentation method on the LGE images, and this mask was then applied on the corresponding T1ρ-maps to calculate T1ρ-values for infarct and remote myocardium [19].
Statistics
Statistical analysis was performed with GraphPad Prism (GraphPad Software, California, United States). Group comparison was performed using a two-way ANOVA analysis, and considered significant at p < 0.05.
Results
Animal study
In 9 out of 16 animals, image quality of the images was sufficient to calculate a T1ρ map. In the other animals, acquisition problems (trigger problems, n = 1) and artifacts (field inhomogeneities (n = 3), foldover-artifacts (n = 2), and motion artifacts due to breathing (n = 1)) led to insufficient image quality to calculate a map.
Mean ejection fraction in the animals 8 weeks after MI was 39.4 ± 8.1%. The T1ρ relaxation time was significantly higher in the infarcted region (57 ± 11 ms), compared to healthy remote myocardium (37 ± 4 ms) (p < 0.0001) (Figure 2A). Histology showed that the amount of fibrosis in the infarct area (44.9 ± 13.2%) was also significantly higher compared to the remote myocardium (6.6 ± 7.1%) (p < 0.0001) (Figure 2B). (see Figure 3).
In vivo T 1ρ relaxation time versus ex vivo histology. A: T1ρ relaxation time measured in vivo in a porcine animal model is significantly higher in infarct area (57 ± 11 ms) compared to healthy myocardium (37 ± 4 ms) (p < 0.001) B: The amount of fibrosis in the infarct area (44.9 ± 13.2%) was also significantly higher compared to the remote myocardium (6.6 ± 7.1%) (p < 0.0001).
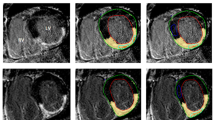
Short axis in vivo T 1ρ -maps of two different animals with corresponding LGE images and ex vivo TTC staining in a porcine animal model 8 weeks after MI. In the top T1ρ-map artefacts can be observed in the left ventricular free wall, which are likely caused by the effect of B0 and B1 inhomogeneities on the spin-lock pulse.
Ex vivo scans of the sectioned heart slices showed that the T1ρ contrast between healthy and infarct myocardium increases with an increasing spin lock amplitude (Figure 4).
Patient study
The patient characteristics of the 21 patients are listed in Table 1. Mean ejection fraction in the patients after MI was 55.7 ± 7.4% (Table 1). Image quality of the T1ρ-maps was good in 9 out of 21 patients, acceptable in 6 patients, and poor in 6 patients. Most important reasons for reduced image quality of the T1ρ-maps were misregistration of the different T1ρ-weighted images, due to a difference in breath hold position for the scans, and fold-over artefacts.
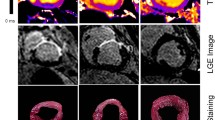
The T1ρ relaxation time was significantly higher in the infarct region (79 ± 11 ms), compared to healthy remote myocardium (54 ± 6 ms), p < 0.0005 (Figure 5). In the healthy young control participants the T1ρ relaxation time was significantly lower compared to the infarct region (50 ± 3 ms), p < 0.0005. The difference between remote myocardium in patients and myocardium in the healthy young control participants was not significant (p = 0.24).
On both LGE and T1ρ 357 segments were scored, of which 31 segments could not be assessed, due to registration artefacts.
The results of the double blinded untrained scoring are listed in Table 2a. In 93 segments enhancement was scored on a LGE image, and in 63 segments on the T1ρ map. Overall overlap on LGE images and T1ρ-maps was 72%.
Results of the unblinded scoring after training can be found in Table 2b. A significant increase in sensitivity was found (0.76 vs. 0.34). In 93 segments enhancement was scored on a LGE image, and in 134 segments on the T1ρ map. Overall overlap on LGE images and T1ρ-maps was 74%.
Discussion
To our knowledge, this is the first report of in vivo detection of chronic myocardial infarction in patients using native T1ρ –mapping without the use of gadolinium contrast agents. Areas of myocardial fibrosis as identified with this approach corresponded reasonably well with conventional LGE images (Figure 6). While the results with the current implementation are promising, sensitivity and specificity is lower compared to the LGE method. We expect that improvements to the implementation will solve this issue, as discussed below. Since T1ρ -mapping requires no contrast agent, it provides a truly noninvasive method, and therefore has the potential to become an alternative to the LGE method in patients with severe renal failure who are unable to receive a contrast agent.
In the animal model we found a significantly higher T1ρ relaxation in the infarct region, compared to healthy myocardium (Figure 2). Histology showed that the collagen fraction in this area was higher compared to macroscopically normal remote myocardium. These results are in accordance with previous findings in other animal studies [12],[15]. Similar to the findings in the animal model, in patients with a chronic myocardial infarction we also found a significant higher T1ρ value in the infarct area. The T1ρ relaxation times found in patients were higher than in the animal model, which could be caused by multiple factors. It is known that the T1ρ relaxation time depends on the strength of the main B0 field, and decreases with a higher B0[20]. Also, the higher amplitude for the spin-lock B1 pulse in the patient study leads to higher values for T1ρ, as we also found in the T1ρ dispersion results (Figure 4). Finally we used an increased trigger interval of 3 beats in the patient study, which enabled a better estimation of the true T1ρ value because of reduced T1 weighting due to incomplete relaxation of the magnetization prior to the next acquisition.
The ex vivo T1ρ dispersion results showed that the T1ρ contrast between healthy myocardium and scar tissue increases with a higher B1 amplitude of the spin-lock pulse (Figure 4). This implies that for T1ρ –mapping we should aim for the highest possible B1 amplitude. However, on a clinical MR scanner the maximum B1 amplitude is limited by the specific absorption rate (SAR) and the performance of the hardware (transmit coil and RF amplifiers). To stay within human SAR limits, a spin-lock amplitude of 500 Hz (11.7 μT) was used at a field strength of 3 T in the animal experiment. The patient study was performed on a 1.5 T system, enabling a higher B1 amplitude for the spin-lock pulse of 750 Hz (17.6 μT), because of lower SAR values and a higher B1 field available. Another reason to perform the patient study on a 1.5 T system, is that artefacts caused by B1 and B0 inhomogeneities, as can be seen in Figure 3, are reduced on a lower field strength. The dispersion data suggests that an even higher B1 amplitude for the spin-lock pulse would generate more contrast between healthy and fibrotic myocardium. New CMR contrast developments such as relaxation along a fictitious field (RAFF) may have the potential to generate more contrast between infarct and remote tissue for a given B1 amplitude and SAR level [21].
Double-blinded qualitative scoring of the LGE images and T1ρ maps in patients was performed to investigate if the native T1ρ maps can be used to assess the presence and location of myocardial scar accurately. We found a 72% agreement between both methods in the patients (Table 2a). However compared with in vivo gold standard LGE imaging, the sensitivity of T1ρ –mapping to detect scar tissue was found to be lower using the present implementation.
The most important reason for the lower sensitivity is that the contrast to noise ratio between healthy myocardium and scar tissue is much higher in LGE imaging. Although the difference in native T1ρ between healthy and infarct tissue is significant, especially smaller infarcts might be more difficult to detect with this method. The infarct size of the patients in this study was small, which can also be concluded from the mean LV ejection fraction of 55.7 ± 7.4% [22].
Important to keep in mind is that the underlying principle to discriminate myocardial scar tissue from normal myocardium with LGE imaging and T1ρ mapping is different. In LGE imaging the difference in contrast agent washout between normal and diseased myocardium is used to identify scar tissue, which reflects changes in perfusion and extracellular volume in the scar area [23]. On the other hand, native T1ρ mapping directly measures the effect of tissue damage and scar tissue formation on the T1ρ relaxation time. T1ρ is known to be sensitive to changes in macromolecular content, and the histology results show that in the infarct region both a significant increase in T1ρ time and fibrosis percentage is found. It is unknown, however, if the increase in T1ρ directly reflects an increase of collagen in scar tissue, or that other changes in tissue composition after MI are involved. Studies in cartilage and protein solutions suggest that other factors such as cellular content and exchange might be involved, since in agarose gels an increase in macromolecule content leads to a decrease in T1ρ time, which is in the opposite direction of our findings [24],[25]. Further research should be performed on the mechanism and relation between myocardial fibrosis formation and the myocardial T1ρ relaxation time.
Another reason for the higher sensitivity of LGE, can be that the radiologist is trained to assess myocardial scar on LGE images, but has no experience in looking at T1ρ maps. After the T1ρ maps were scored double blinded, the radiologist was trained to look at T1ρ maps, and scored again with the LGE images shown alongside. This resulted in a significant increase of sensitivity for the detection of infarct area with T1ρ mapping compared to LGE imaging. We also observed that endocardial infarcts were more difficult to detect on T1ρ maps, since partial volume effects, combined with the high T1ρ relaxation time of the blood, make it difficult to distinguish the transition from myocardium to blood on a T1ρ map. Furthermore, since we used multiple breath holds to calculate a T1ρ map, misregistration between the different breath hold positions leads to problems with the calculation of the T1ρ map at the edges of the myocardium.
Future work should aim to overcome these limitations that reduced image quality and assessability of the T1ρ maps in our study. Most important step is the development of a single breath hold T1ρ mapping sequence to obtain a high quality T1ρ map, without registration errors due to multiple breath hold acquisitions. This requires faster cardiac T1ρ mapping sequences, using acceleration methods. Furthermore, a black-blood readout will enable the detection of an endocardial infarct close to the blood, by reducing partial volume effects.
One of the main drawbacks of LGE imaging is the lack of the ability to measure myocardial fibrosis quantitatively. Currently there is a lot of interest in quantitative imaging of myocardial tissue to overcome these limitations, by measuring quantitative contrast enhanced T1-maps and extracellular volume (ECV)-maps [26],[27]. We believe that native T1ρ –mapping could provide additional quantitative information on myocardial fibrosis in cardiomyopathies, also in patients with diffuse interstitial myocardial fibrosis. Native T1ρ –mapping requires no separate pre- and post-contrast scan, no hematocrit measurement, and is therefore easier to incorporate in a clinical protocol, compared to ECV-mapping. Here we have shown the first evidence that T1ρ mapping can provide quantitative information on myocardial fibrosis in patients with a chronic myocardial infarction. Though speculative, the finding that the remote myocardium of the patients was about 1 SD above the T1ρ value of the healthy subjects, may suggest a slight increase in diffuse interstitial fibrosis in this area, which could become significant if a larger group with more statistical power would be studied. Future work should focus on further validation of the relation between myocardial fibrosis and T1ρ, and on the relation with native and contrast-enhanced T1 and ECV-mapping.
Conclusion
In conclusion, we have demonstrated the feasibility of T1ρ mapping for infarct detection, without the use of an exogenous contrast agent, in patients with a chronic myocardial infarction. Although the sensitivity of T1ρ mapping is lower than LGE imaging, there is room for improvements on the T1ρ mapping sequence that could provide a higher sensitivity and specificity. We believe that T1ρ mapping could provide additional information on myocardial tissue characteristics, and be used in the clinic along with quantitative T1, T2 and ECV mapping methods, to further understand the development of ischemic and non-ischemic cardiomyopathies.
Abbreviations
- MI:
-
Myocardial infarction
- LGE:
-
Late gadolinium enhancement
- CA:
-
Contrast agent
- CMR:
-
Cardiovascular Magnetic Resonance
- LAD:
-
Left anterior descending
- NSA:
-
Number of signals averaged
- SL:
-
Spin lock
- FOV:
-
Field of view
- SSFP:
-
Steady-state free precession
- TE:
-
Echo time
- TR:
-
Repetition time
References
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM: The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000, 343: 1445-53. 10.1056/NEJM200011163432003.
Kwong RY, Chan AK, Brown K a, Chan CW, Reynolds HG, Tsang S, Davis RB: Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006, 113: 2733-43. 10.1161/CIRCULATIONAHA.105.570648.
Müller K a L, Müller I, Kramer U, Kandolf R, Gawaz M, Bauer A, Zuern CS: Prognostic Value of Contrast-enhanced Cardiac Magnetic Resonance Imaging in Patients with Newly Diagnosed Non-Ischemic Cardiomyopathy: Cohort Study. PLoS One. 2013, 8: e57077-10.1371/journal.pone.0057077.
El Aidi H, Adams A, Moons KGM, Den Ruijter HM, Mali WPTM, Doevendans PA, Nagel E, Schalla S, Bots ML, Leiner T: Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease: a systematic review of prognostic studies. J Am Coll Cardiol. 2014, 63: 1031-45. 10.1016/j.jacc.2013.11.048.
Nacif MS, Arai AE, Lima JAC, Bluemke DA: Gadolinium-enhanced cardiovascular magnetic resonance: administered dose in relationship to United States Food and Drug Administration (FDA) guidelines. J Cardiovasc Magn Reson. 2012, 14: 18-
Bellin M-F, Van Der Molen AJ: Extracellular gadolinium-based contrast media: an overview. Eur J Radiol. 2008, 66: 160-7. 10.1016/j.ejrad.2008.01.023.
Raisch DW, Garg V, Arabyat R, Shen X, Edwards BJ, Miller FH, McKoy JM, Nardone B, West DP: Anaphylaxis associated with gadolinium-based contrast agents: data from the Food and Drug Administration’s adverse event reporting system and review of case reports in the literature. Expert Opin Drug Saf. 2014, 13: 15-23. 10.1517/14740338.2013.832752.
Zou Z, Zhang HL, Roditi GH, Leiner T, Kucharczyk W, Prince MR: Nephrogenic systemic fibrosis: review of 370 biopsy-confirmed cases. JACC Cardiovasc Imaging. 2011, 4: 1206-16. 10.1016/j.jcmg.2011.08.013.
Van Oorschot JWM, Gho JMIH, Van Hout GPJ, Froeling M, Jansen of Lorkeers SJ, Hoefer IE, Doevendans PA, Luijten PR, Chamuleau SAJ, Zwanenburg JJM. Endogenous contrast MRI of cardiac fibrosis: Beyond late gadolinium enhancement.J Magn Reson Imaging. 2014; doi: 10.1002/jmri.24715. [Epub ahead of print].,
Menezes NM, Gray ML, Hartke JR, Burstein D: T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004, 51: 503-9. 10.1002/mrm.10710.
Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, Ries MD, Horvai A, Link TM, Majumdar S: Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011, 29: 324-34. 10.1016/j.mri.2010.09.004.
Witschey WR, Zsido GA, Koomalsingh K, Kondo N, Minakawa M, Shuto T, McGarvey JR, Levack M a, Contijoch F, Pilla JJ, Gorman JH, Gorman RC: In vivo chronic myocardial infarction characterization by spin locked cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012, 14: 37-10.1186/1532-429X-14-37.
Muthupillai R, Flamm SD, Wilson JM, Pettigrew RI: Radiology Acute Myocardial Infarction: Tissue Characterization with T1rho -weighted MR Imaging — Initial Experience. Radiology. 2004, 232 (2): 606-610. 10.1148/radiol.2322030334. Epub 2004 Jun 23
Witschey W, Pilla J, Kevin FG, Koomalsingh : Rotating frame spin lattice relaxation in a swine model of chronic, left ventricular myocardial infarction. Magn Reson Med. 2010, 64: 1453-60. 10.1002/mrm.22543.
Musthafa H-SN, Dragneva G, Lottonen L, Merentie M, Petrov L, Heikura T, Ylä-Herttuala E, Ylä-Herttuala S, Gröhn O, Liimatainen T: Longitudinal rotating frame relaxation time measurements in infarcted mouse myocardium in vivo. Magn Reson Med. 2012, 000: 1-7.
Borthakur A, Charagundla SR, Wheaton A, Reddy R: T1rho-weighted MRI using a surface coil to transmit spin-lock pulses. J Magn Reson. 2004, 167: 306-16. 10.1016/j.jmr.2004.01.007.
Witschey WR, Borthakur A, Elliott MA, Metllon E, Niyogi S, Wallman DJ, Wang C, Reddy R: Artifacts in T1ρ-Weighted Imaging: Compensation for B1 and B0 Field Imperfections. J Magn Reson. 2007, 186 (1): 75-85. 10.1016/j.jmr.2007.01.015. Epub 2007 Jan 26.
Cerqueira MD: Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart: A Statement for Healthcare Professionals From the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002, 105: 539-42. 10.1161/hc0402.102975.
Hsu L-Y, Natanzon A, Kellman P, Hirsch GA, Aletras AH, Arai AE: Quantitative myocardial infarction on delayed enhancement MRI. Part I: Animal validation of an automated feature analysis and combined thresholding infarct sizing algorithm. J Magn Reson Imaging. 2006, 23: 298-308. 10.1002/jmri.20496.
Mäkelä HI, De Vita E, Gröhn OHJ, Kettunen MI, Kavec M, Lythgoe M, Garwood M, Ordidge R, Kauppinen RA: B0 dependence of the on-resonance longitudinal relaxation time in the rotating frame (T1rho) in protein phantoms and rat brain in vivo. Magn Reson Med. 2004, 51: 4-8. 10.1002/mrm.10669.
Liimatainen T, Sorce DJ, O’Connell R, Garwood M, Michaeli S: MRI contrast from relaxation along a fictitious field (RAFF). Magn Reson Med. 2010, 64: 983-94. 10.1002/mrm.22372.
Burns RJ, Gibbons RJ, Yi Q, Roberts RS, Miller TD, Schaer GL, Anderson JL, Yusuf S: The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol. 2002, 39: 30-6. 10.1016/S0735-1097(01)01711-9.
Kim RJ, Fieno DS, Parrish TB, Harris K, Chen E-L, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM: Relationship of MRI Delayed Contrast Enhancement to Irreversible Injury, Infarct Age, and Contractile Function. Circulation. 1999, 100: 1992-2002. 10.1161/01.CIR.100.19.1992.
Andrasko J: Water in agarose gels studied by nuclear magnetic resonance relaxation in the rotating frame. Biophys J. 1975, 15: 1235-43. 10.1016/S0006-3495(75)85896-6.
Virta A, Komu M, Kormano M: T1rho of protein solutions at very low fields: dependence on molecular weight, concentration, and structure. Magn Reson Med. 1997, 37: 53-7. 10.1002/mrm.1910370109.
White SK, Sado DM, Fontana M, Banypersad SM, Maestrini V, Flett AS, Piechnik SK, Robson MD, Hausenloy DJ, Sheikh AM, Hawkins PN, Moon JC: T1 mapping for myocardial extracellular volume measurement by CMR: bolus only versus primed infusion technique. JACC Cardiovasc Imaging. 2013, 6: 955-62. 10.1016/j.jcmg.2013.01.011.
Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, Francis JM, Karamitsos TD, Prendergast BD, Robson MD, Neubauer S, Moon JC, Myerson SG: Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013, 99 (13): 932-937. 10.1136/heartjnl-2012-303052. doi: 10.1136/heartjnl-2012-303052. Epub 2013 Jan 24
Acknowledgements
This research forms part of the Project P1.04 SMARTCARE of the research program of the BioMedical Materials Institute, co-funded by the Dutch Ministry of Economic Affairs, Agriculture and Innovation. The financial contribution of the Nederlandse Hartstichting is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
FV is an employee of Philips Healthcare, Best, The Netherlands.
Authors’ contributions
JG, SJ, SC performed the LAD occlusion and histology. JvO, MF, JZ, FV performed the animal CMR experiments and processed the CMR data. JvO, HeA, JZ, FV performed the patient CMR experiments and processed the CMR data. TL scored the CMR data. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
van Oorschot, J.W., El Aidi, H., Jansen of Lorkeers, S.J. et al. Endogenous assessment of chronic myocardial infarction with T1ρ-mapping in patients. J Cardiovasc Magn Reson 16, 104 (2014). https://doi.org/10.1186/s12968-014-0104-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12968-014-0104-y