Abstract
Background
Pericallosal lipomas are often associated with corpus callosum dysgenesis. The diagnosis of lipoma, suggested on ultrasonography, relies on the classic T1 hyperintensity on magnetic resonance imaging (MRI). However, this feature may be absent prenatally.
Objective
Our objective was to study the changes of T1 intensity in fetal lipomas with comparison to postnatal/postmortem data and to assess the factors influencing the signal variations of pericallosal lipomas on prenatal MRI.
Materials and methods
Patients with callosum dysgenesis and interhemispheric hyperechogenicity suggestive of a pericallosal lipoma with available postnatal or postmortem data were included. Gestational age, lipoma size and pattern, corpus callosum size and changes in fetal fat T1 intensity were recorded. Comparison with postmortem neuropathology was available for one fetus.
Results
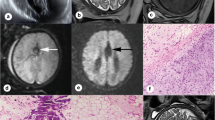
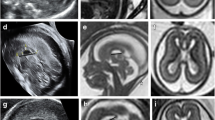
Eleven patients with callosum dysgenesis and pericallosal lipomas (seven curvilinear and four tubulonodular) were included. All MRI scans were performed in the third trimester. Curvilinear lipomas were thinner and six cases were associated with prenatal T1 iso-intensity. Typical T1 hyperintensity appeared on postnatal MRI only. All tubulonodular lipomas were much larger and showed prenatal T1 hyperintensity. In two patients, the lipoma increased in size on postnatal MRI.
Conclusion
The type and size of a lipoma influence T1 prenatal intensity. Absence of T1 intensity was observed in curvilinear lipomas only. Curvilinear lipomas are much thinner. Changes in T1 intensity may also be related to fat maturation within the lipoma and, subsequently, to gestational age. In the case of callosum dysgenesis, absence of prenatal T1 pericallosal hyperintensity should not exclude the diagnosis of pericallosal lipoma.




Similar content being viewed by others
References
Truwit CL, Barkovich AJ (1990) Pathogenesis of intracranial lipoma: an MR study in 42 patients. AJR Am J Roentgenol 155:855–864
Yildiz H, Hakyemez B, Koroglu M et al (2006) Intracranial lipomas: importance of localization. Neuroradiology 48:1–7
Jabot G, Stoquart-Elsankari S, Saliou G et al (2009) Intracranial lipomas: clinical appearances on neuroimaging and clinical significance. J Neurol 256:851–855
Dean B, Drayer BP, Beresini DC et al (1988) MR imaging of pericallosal lipoma. AJNR Am J Neuroradiol 9:929–931
Erro Aguirre ME, de Lapiscina EHM (2014) Lipoma: an overview. In: Hayat M (ed) Tumors of the central nervous system. Springer, Dordrecht, pp 223–229
Ickowitz V, Eurin D, Rypens F et al (2001) Prenatal diagnosis and postnatal follow-up of pericallosal lipoma: report of seven new cases. AJNR Am J Neuroradiol 22:767–772
Garel C (2004) Pathology of the midline. In: Garel C (ed) MRI of the fetal brain: normal development and cerebral pathologies. Springer Verlag, Berlin, pp 131–150
Atallah A, Lacalm A, Massoud M et al (2017) Limitations and pitfalls in prenatal diagnosis of pericallosal curvilinear lipoma based on a specific imaging pattern. Ultrasound Obstet Gynecol. https://doi.org/10.1002/uog.17400
Garel C, Moutard ML (2014) Main congenital cerebral anomalies: how prenatal imaging aids counseling. Fetal Diagn Ther 35:229–239
Tart RP, Quisling RG (1991) Curvilinear and tubulonodular varieties of lipoma of the corpus callosum: an MR and CT study. J Comput Assist Tomogr 15:805–810
Yilmaz MB, Genc A, Egemen E et al (2016) Pericallosal lipomas: a series of 10 cases with clinical and radiological features. Turk Neurosurg 26:364–368
Cignini P, Padula F, Giorlandino M et al (2014) Reference charts for fetal corpus callosum length: a prospective cross-sectional study of 2950 fetuses. J Ultrasound Med 33:1065–1078
Garel C, Cont I, Alberti C et al (2011) Biometry of the corpus callosum in children: MR imaging reference data. AJNR Am J Neuroradiol 32:1436–1443
Tilea B, Alberti C, Adamsbaum C et al (2009) Cerebral biometry in fetal magnetic resonance imaging: new reference data. Ultrasound Obstet Gynecol 33:173–181
Atanassova P (2001) Immunohistochemical expression of S-100 protein in human embryonal fat cells. Cells Tissues Organs 169:355–360
Niwa T, de Vries LS, Manten GT et al (2016) Interhemispheric lipoma, callosal anomaly, and malformations of cortical development: a case series. Neuropediatrics 47:115–118
Pierre-Kahn A, Zerah M, Renier D et al (1997) Congenital lumbosacral lipomas. Childs Nerv Syst 13:298–334
Frontini A, Cinti S (2010) Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab 11:253–256
Klingenspor M, Fromme T (2012) Brown adipose tissue. In: Symonds ME (ed) Adipose tissue biology. Springer, New York, pp 39–69
Saely CH, Geiger K, Drexel H (2012) Brown versus white adipose tissue: a mini-review. Gerontology 58:15–23
Symonds ME, Pope M, Budge H (2015) The ontogeny of brown adipose tissue. Annu Rev Nutr 35:295–320
Blondiaux E, Chougar L, Gelot A et al (2017) Normal MRI developmental patterns of fetal fat intensity. Pediatr Radiol. https://doi.org/10.1007/s00247-017-4038-z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Chougar, L., Blondiaux, E., Moutard, ML. et al. Variability of T1-weighted signal intensity of pericallosal lipomas in the fetus. Pediatr Radiol 48, 383–391 (2018). https://doi.org/10.1007/s00247-017-4028-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-017-4028-1