Abstract
Background
There is evidence of microstructural changes in normal-appearing white matter of patients with tuberous sclerosis complex.
Objective
To evaluate major white matter tracts in children with tuberous sclerosis complex using tract-based spatial statistics diffusion tensor imaging (DTI) analysis.
Materials and methods
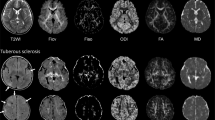
Eight children (mean age ± standard deviation: 8.5 ± 5.5 years) with an established diagnosis of tuberous sclerosis complex and 8 age-matched controls were studied. The imaging protocol consisted of T1-weighted high-resolution 3-D spoiled gradient-echo sequence and a spin-echo, echo-planar diffusion-weighted sequence. Differences in the diffusion indices were evaluated using tract-based spatial statistics.
Results
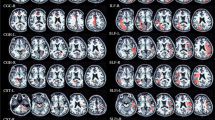
Tract-based spatial statistics showed increased axial diffusivity in the children with tuberous sclerosis complex in the superior and anterior corona radiata, the superior longitudinal fascicle, the inferior fronto-occipital fascicle, the uncinate fascicle and the anterior thalamic radiation. No significant differences were observed in fractional anisotropy, mean diffusivity and radial diffusivity between patients and control subjects. No difference was found in the diffusion indices between the baseline and follow-up examination in the patient group.
Conclusion
Patients with tuberous sclerosis complex have increased axial diffusivity in major white matter tracts, probably related to reduced axonal integrity.

Similar content being viewed by others
References
Crino PB, Nathanson KL, Henske EP (2006) The tuberous sclerosis complex. N Engl J Med 355:1345–1356
Ess KC (2006) The neurobiology of tuberous sclerosis complex. Semin Pediatr Neurol 13:37–42
Dabora S, Jozwiak S, Neal Franz D et al (2001) Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 68:64–80
Curatolo P, Bombardieri R, Jozwiak S (2008) Tuberous sclerosis. Lancet 372:657–668
Meikle L, Talos DM, Onda H et al (2007) A mouse model of tuberous sclerosis: neuronal loss of Tsc1causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci 27:5546–5558
Schaefer PW, Grant PE, Gonzalez RG (2000) Diffusion-weighted MR imaging of the brain. Radiology 217:331–345
Astrakas LG, Argyropoulou MI (2010) Shifting from region of interest (ROI) to voxel-based analysis in human brain mapping. Pediatr Radiol 40:1857–1867
Karadag D, Mentzel H-J, Gullmar D et al (2005) Diffusion tensor imaging in children and adolescents with tuberous sclerosis. Pediatr Radiol 35:980–983
Piao C, Yu A, Li K et al (2009) Cerebral diffusion tensor imaging in tuberous sclerosis. Eur J Radiol 71:249–252
Makki MI, Chugani DC, Janisse J et al (2007) Characteristics of abnormal diffusivity in normal-appearing white matter investigated with diffusion tensor MR imaging in tuberous sclerosis complex. AJNR Am J Neuroradiol 28:1662–1667
Simao G, Raybaud C, Chuang S et al (2010) Diffusion tensor imaging of commissural and projection white matter in tuberous sclerosis complex and correlation with tuber load. AJNR Am J Neuroradiol 31:1273–1277
Krishnan M, Commowick O, Jeste S et al (2010) Diffusion features of white matter in tuberous sclerosis with tractography. Pediatr Neurol 42:101–106
Arulrajah S, Ertan G, Jordan L et al (2009) Magnetic resonance imaging and diffusion-weighted imaging of normal-appearing white matter in children and young adults with tuberous sclerosis complex. Neuroradiology 51:781–786
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Wong AM, Wang H-S, Schwartz ES et al (2013) Cerebral diffusion tensor MR tractography in tuberous sclerosis complex: correlation with neurologic severity and tract-base spatial statistical analysis. AJNR Am J Neuroradiol 34:1829–1835
Roach ES, Gomez MR, Northrup H (1998) Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol 13:624–628
Northrup H, Krueger DA (2013) Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 49:243–254
Boer K, Troost D, Jansen F et al (2008) Clinicopathological and immunohistochemical findings in an autopsy case of tuberous sclerosis complex. Neuropathology 28:577–590
Qiu D, Tan L-H, Zhou K et al (2008) Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage 41:223–232
Zivadinov R, Shucard GL, Hussein S et al (2013) Multimodal imaging in systemic lupus erythematosus patients with diffuse neuropsychiatric involvement. Lupus 22:675–683
Schwartz E, Cooper E, Fan Y et al (2005) MRI diffusion coeficients in spinal cord correlate with axon morphometry. Brain Imaging 16:73–76
Kinoshita Y, Ohnishi A, Kohshi K et al (1999) Apparent diffusion coefficient on rat brain and nerves intoxicated with methylmercury. Environ Res 80:348–354
Choi YJ, Di Nardo A, Kramvis I et al (2008) Tuberous sclerosis complex proteins control axon formation. Genes Dev 22:2485–2495
Song SK, Sun SW, Ju WK et al (2003) Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20:1714–1722
Budde MD, Kim JH, Liang HF et al (2008) Axonal injury detected by in vivo diffusion tensor imaging correlates with neurological disability in a mouse model of multiple sclerosis. NMR Biomed 21:589–597
Hofling AA, Kim JH, Fantz CR et al (2009) Diffusion tensor imaging detects axonal injury and demyelination in the spinal cord and cranial nerves of a murine model of globoid cell leukodystrophy. NMR Biomed 22:1100–1106
Roosendaal SD, Geurts JJ, Vrenken H et al (2009) Regional DTI differences in multiple sclerosis patients. Neuroimage 44:1397–1403
Metwalli NS, Benatar M, Nair G et al (2010) Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Res 12:156–164
Hasan KM, Halphen C, Boska MD et al (2008) Diffusion tensor metrics, T2 relaxation, and volumetry of the naturally aging human caudate nuclei in healthy young and middle-aged adults: possible implications for the neurobiology of human brain aging and disease. Magn Reson Med 59:7–13
Bergers E, Bot JC, De Groot CJ et al (2002) Axonal damage in the spinal cord of MS patients occurs largely independent of T2 MRI lesions. Neurology 59:1766–1771
Trapp BD, Peterson J, Ransohoff RM et al (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338:278–285
Kamali A, Flanders AE, Brody J et al (2014) Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct 219:269–281
Tang YY, Lu Q, Geng X et al (2010) Short-term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci U S A 107:15649–15652
Radoeva P, Coman I, Antshel KΜ et al (2012) Atlas-based white matter analysis in individuals with velo-cardio-facial syndrome (22q11.2 deletion syndrome) and unaffected siblings. Behav Brain Funct. doi:10.1186/1744-9081-8-38
Yin X, Han Y, Ge H et al (2013) Inferior frontal white matter asymmetry correlates with executive control of attention. Hum Brain Mapp 34:796–813
Ge H, Yin X, Xu J et al (2013) Fiber pathways of attention subnetworks revealed with tract-based spatial statistics (TBSS) and probabilistic tractography. PLoS One 8, e78831
Martino J, Brogna C, Robles SG et al (2010) Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex 46:691–699
Von Der Heide RJ, Skipper LM, Klobusicky E et al (2013) Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain 136:1692–1707
Nair A, Treiber JM, Shukla DK et al (2013) Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain 136:1942–1955
Pulsipher DT, Seidenberg M, Guidotti L et al (2009) Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia 50:1210–1219
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Zikou, A.K., Xydis, V.G., Astrakas, L.G. et al. Diffusion tensor imaging in children with tuberous sclerosis complex: tract-based spatial statistics assessment of brain microstructural changes. Pediatr Radiol 46, 1158–1164 (2016). https://doi.org/10.1007/s00247-016-3582-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-016-3582-2