Abstract
Background
Diffusion-weighted imaging (DWI) is important in the assessment of fetal brain development. However, it is clinically challenging and time-consuming to prepare neuromorphological examinations to assess real brain age and to detect abnormalities.
Objective
To demonstrate that the Gini coefficient can be a simple, intuitive parameter for modelling fetal brain development.
Materials and methods
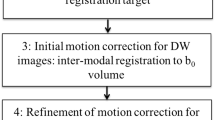
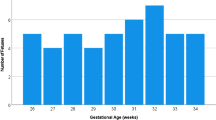
Postmortem fetal specimens(n = 28) were evaluated by diffusion-weighted imaging (DWI) on a 3-T MRI scanner using 60 directions, 0.7-mm isotropic voxels and b-values of 0, 150, 1,600 s/mm2. Constrained spherical deconvolution (CSD) was used as the local diffusion model. Fractional anisotropy (FA), apparent diffusion coefficient (ADC) and complexity (CX) maps were generated. CX was defined as a novel diffusion metric. On the basis of those three parameters, the Gini coefficient was calculated.
Results
Study of fetal brain development in postmortem specimens was feasible using DWI. The Gini coefficient could be calculated for the combination of the three diffusion parameters. This multidimensional Gini coefficient correlated well with age (Adjusted R2 = 0.59) between the ages of 17 and 26 gestational weeks.
Conclusions
We propose a new method that uses an economics concept, the Gini coefficient, to describe the whole brain with one simple and intuitive measure, which can be used to assess the brain’s developmental state.





Similar content being viewed by others
References
Blondiaux E, Garel C (2013) Fetal cerebral imaging - ultrasound vs. MRI: an update. Acta Radiol 54:1046–1054
Guihard-Costa AM, Larroche JC, Droullé P (1995) Fetal biometry. Growth charts for practical use in fetopathology and antenatal ultrasonography. Fetal Diagn Ther 10:211–278
Guihard-Costa AM, Larroche JC (1992) Growth velocity of some fetal parameters. I: Brain weight and brain dimensions. Biol Neonate 62:309–316
Habas PA, Kim K, Rousseau F et al (2010) Atlas-based segmentation of developing tissues in the human brain with quantitative validation in young fetuses. Hum Brain Mapp 31:1348–1358
Gholipour A, Estroff JA, Barnewolt CE et al (2011) Fetal brain volumetry through MRI volumetric reconstruction and segmentation. Int J Comput Assist Radiol Surg 6:329–339
Chi JG, Dooling EC, Gilles FH (1977) Gyral development of the human brain. Ann Neurol 1:86–93
Zhang Z, Liu S, Lin X et al (2011) Development of laminar organization of the fetal cerebrum at 3.0 T and 7.0 T: a postmortem MRI study. Neuroradiology 53:177–184
Huang H, Jeon T, Sedmak G et al (2012) Coupling diffusion imaging with histological and gene expression analysis to examine the dynamics of cortical areas across the fetal period of human brain development. Cereb Cortex 23:2620–2631
Bendersky M, Musolino PL, Rugilo C et al (2006) Normal anatomy of the developing fetal brain. Ex vivo anatomical-magnetic resonance imaging correlation. J Neurol Sci 250:20–26
Bendersky M, Tamer I, Van Der Velde J et al (2008) Prenatal cerebral magnetic resonance imaging. J Neurol Sci 275:37–41
Gini C (1912) (italian) Variabilità e mutabilità (Variability and Mutability). C. Cuppini, Bologna
http://en.wikipedia.org/wiki/Gini_coefficient. Accessed 27 October 2013
Jacobson M, Rao MS (2005) Developmental neurobiology. Kluwer Academic/Plenum, New York
Kasprian G, Brugger PC, Weber M et al (2008) In utero tractography of fetal white matter development. Neuroimage 43:213–224
Vasung L, Huang H, Jovanov-Milošević N et al (2010) Development of axonal pathways in the human fetal fronto-limbic brain: histochemical characterization and diffusion tensor imaging. J Anat 217:400–417
Kasprian G, Del Río M, Prayer D (2010) Fetal diffusion imaging: pearls and solutions. Top Magn Reson Imaging 21:387–394
Jenkinson M, Bannister P, Brady JM et al (2002) Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Tournier JD, Calamantea F, Connelly A (2007) Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. Neuroimage 35:1459–1472
Tournier JD, Calamante F, Connelly A (2012) MRtrix: Diffusion tractography in crossing fiber regions. Int J Imaging Syst Technol 22:53–66
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Res 111:209–219
Ropele S, Seewann A, Gouw AA et al (2009) Quantitation of brain tissue changes associated with white matter hyperintensities by diffusion-weighted and magnetization transfer imaging: The LADIS (leukoaraiosis and disability in the elderly) study. J Magn Res Imag 29:268–274
Aliotta R, Cox JL, Donohue K et al (2012) Tract-based spatial statistics analysis of diffusion-tensor imaging data in pediatric- and adult-onset multiple sclerosis. Hum Brain Mapp 35:53–60
Widjaja E, Geibprasert S, Mahmoodabadi SZ et al (2010) Alteration of human fetal subplate layer and intermediate zone during normal development on MR and diffusion tensor imaging. AJNR Am J Neuroradiol 31:1091–1099
Jones DK (2010) Precision and accuracy in diffusion tensor magnetic resonance imaging. Top Magn Res Imag 21:87–99
Riffert T, Anwander A, Knoesche TR (2012) Characterizing properties by fiber bundle parameters derived from the fODF, Poster presented at the 18th Annual Meeting of the Organization for Human Brain Mapping, Beijing, China
Bowman A, Azzalini A Smoothing methods for nonparametric regression and density estimation, http://cran.r-project.org/web/packages/sm/index.html, version 5.4, accessed 02 May 2013
Bowman AW, Azzalini A (1997) Applied smoothing techniques for data analysis: the kernel approach with S-plus illustrations. Oxford University Press, Oxford
Handcock MS, http://cran.r-project.org/web/packages/reldist/, Relative Distribution Methods, version 1.6-2, accessed 02 May 2013
Gastwirth JL (1972) The estimation of the Lorenz Curve and Gini Index. Rev Econ and Stat 54:306
Verwer RW, Hermens WT, Dijkhuizen P et al (2002) Cells in human postmortem brain tissue slices remain alive for several weeks in culture. FASEB J 16:54–60
Stan AD, Ghose S, Gao XM et al (2006) Human postmortem tissue: what quality markers matter? Brain Res 1123:1–11
Thayyil S, Sebire NJ, Chitty LS et al (2011) Post mortem magnetic resonance imaging in the fetus, infant and child: a comparative study with conventional autopsy (MaRIAS Protocol). BMC Pediatr 11:120
Miller KL, Stagg CJ, Douaud G et al (2011) Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage 57:167–181
van der Made AD, Maas M, Beenen LFM et al (2012) Postmortem imaging exposed: an aid in MR imaging of musculoskeletal structures. Skelet Radiol 42:467–472
Girard NJ, Chaumoitre K (2012) The brain in the belly: what and how of fetal neuroimaging? J Magn Reson Imaging 36:788–804
Hasan KM, Walimuni IS, Abid H et al (2011) A review of diffusion tensor magnetic resonance imaging computational methods and software tools. Comput Biol Med 41:1062–1072
Jeurissen B, Leemans A, Jones DK et al (2011) Probabilistic fiber tracking using the residual bootstrap with constrained spherical deconvolution. Hum Brain Mapp 32:461–479
Gupta RK, Hasan KM, Trivedi R et al (2005) Diffusion tensor imaging of the developing human cerebrum. J Neurosci Res 81:172–178
Setsompop K, Kimmlingen R, Eberlein E et al (2013) Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. Neuroimage 80:220–233
Tisdall MD, Hess AT, Reuter M et al (2012) Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med 68:389–399
Kim K, Habas PA, Rousseau F et al (2010) Intersection based motion correction of multislice MRI for 3-D in utero fetal brain image formation. IEEE Trans Med Imaging 29:146–158
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viehweger, A., Riffert, T., Dhital, B. et al. The Gini coefficient: a methodological pilot study to assess fetal brain development employing postmortem diffusion MRI. Pediatr Radiol 44, 1290–1301 (2014). https://doi.org/10.1007/s00247-014-3002-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-014-3002-4